Abstract
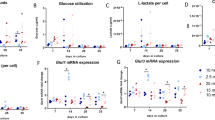
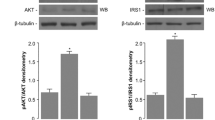
Bone disease as a consequence of diabetes mellitus (DM) is not fully understood. The effects of high glucose (30 mM), high insulin (50 nM), or mannitol (30 mM; osmotic control) were evaluated on MC3T3-E1 cells (osteoblasts) in vitro. The mRNA and protein levels of parathyroid hormone (PTH) receptor (PTH1R), collagen I, RANKL, osteoprotegerin (OPG), alkaline phosphatase (ALP), and glucose transporter (GLUT1) were estimated by real-time polymerase chain reaction or Western blotting. The mineralization capacity was analyzed by von Kossa staining. High glucose induced overexpression of RANKL (2×) and OPG (30×), suggesting that RANKL-induced osteoclast activity might not be a dominant mechanism of bone disease in DM, since this increase was followed by increased OPG. Collagen I increased by 12×, indicating an excess of organic matrix production. The expression of ALP decreased by 50 %, indicating a deficit in mineralization capacity, confirmed by von Kossa staining. Mannitol induced similar effects as glucose suggesting that extracellular hyperosmolarity was able to stimulate organic matrix production. GLUT1 expression was not altered, and insulin did not reverse most of the effects of glucose, suggesting that glucose uptake by osteoblasts was not altered by high glucose. The data suggest that the bone fragility typical of DM is not a consequence of excessive bone reabsorption but is instead attributable to a defect in organic matrix mineralization. The heightened increase in OPG versus RANKL might cause a decrease in the bone-remodeling cycle. Osteoblasts appear to be more sensitive to extracellular hypertonicity than to the intracellular metabolic effects of hyperglycemia.





Similar content being viewed by others
References
Andress DL (2008) Adynamic bone in patients with chronic kidney disease. Kidney Int 73:1345–1354
Balint E, Szabo P, Marshall CF, Sprague SM (2001) Glucose-induced inhibition of in vitro bone mineralization. Bone 28:21–28
Botolin S, McCabe LR (2006) Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem 99:411–424
Botushanov NP, Orbetzova MM (2009) Bone mineral density and fracture risk in patients with type 1 and type 2 diabetes mellitus. Folia Med (Plovdiv) 51:12–17
Brancaccio D, Cozzolino M (2011) CKD-MBD: an endless story. J Nephrol 24 (Suppl 18):S42–S48
Christenson RH (1997) Biochemical markers of bone metabolism: an overview. Clin Biochem 30:573–593
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3 (Suppl 3):S131–S139
Covic A, Apetrii M (2011) Vitamin D receptor activation: clinical outcomes. Contrib Nephrol 171:166–171
Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG et al (2007) Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167:133–139
Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC et al (2006) Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int 79:102–111
Fried LF, Shlipak MG, Stehman-Breen C, Mittalhenkle A, Seliger S et al (2006) Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 61:743–748
Garcia-Hernandez A, Arzate H, Gil-Chavarria I, Rojo R, Moreno-Fierros L (2012) High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone 50:276–288
Grigoropoulou P, Eleftheriadou I, Zoupas C, Tentolouris N (2011) The role of the osteoprotegerin/RANKL/RANK system in diabetic vascular disease. Curr Med Chem 18:4813–4819
Isidro ML, Ruano B (2010) Bone disease in diabetes. Curr Diabetes Rev 6:144–155
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE et al (2003) Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol 149:39–42
Kumagai AK (1999) Glucose transport in brain and retina: implications in the management and complications of diabetes. Diabetes Metab Res Rev 15:261–273
Leslie WD, Rubin MR, Schwartz AV, Kanis JA (2012) Type 2 diabetes and bone. J Bone Miner Res 27:2231–2237
Lorenzo Sellares V, Torregrosa V (2008) Changes in mineral metabolism in stage 3, 4, and 5 chronic kidney disease (not on dialysis). Nefrologia 28 (Suppl 3):67–78
Lozano D, de Castro LF, Dapia S, Andrade-Zapata I, Manzarbeitia F et al (2009) Role of parathyroid hormone-related protein in the decreased osteoblast function in diabetes related osteopenia. Endocrinology 150:2027–2035
Luppen CA, Smith E, Spevak L, Boskey AL, Frenkel B (2003) Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res 18:1186–1197
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N et al (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Montagnani A, Gonnelli S, Alessandri M, Nuti R (2011) Osteoporosis and risk of fracture in patients with diabetes: an update. Aging Clin Exp Res 23:84–90
Moorthi RN, Moe SM (2011) CKD-mineral and bone disorder: core curriculum 2011. Am J Kidney Dis 58:1022–1036
Moseley KF (2012) Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes 19:128–135
Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR et al (2010) Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone 48:733–740
Olesen P, Ledet T, Rasmussen LM (2005) Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia 48:561–568
Perez-Castrillon JL, De Luis D, Martin-Escudero JC, Asensio T, del Amo R et al (2004) Non-insulin-dependent diabetes, bone mineral density, and cardiovascular risk factors. J Diabetes Complicat 18:317–321
Picton ML, Moore PR, Mawer EB, Houghton D, Freemont AJ et al (2000) Down-regulation of human osteoblast PTH/PTHrP receptor mRNA in end-stage renal failure. Kidney Int 58:1440–1449
Rakic V, Davis WA, Chubb SA, Islam FM, Prince RL et al (2006) Bone mineral density and its determinants in diabetes: the Fremantle Diabetes Study. Diabetologia 49:863–871
Sardone LD, Renlund R, Willett TL, Fantus IG, Grynpas MD (2011) Effect of rosiglitazone on bone quality in a rat model of insulin resistance and osteoporosis. Diabetes 60:3271–3278
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Terkeltaub RA (2001) Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol 281:C1–C11
Thomas DM, Maher F, Rogers SD, Best JD (1996) Expression and regulation by insulin of GLUT 3 in UMR 106-01, a clonal rat osteosarcoma cell line. Biochem Biophys Res Commun 218:789–793
Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE et al (2008) High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone 42:1122–1130
Wu YY, Yu T, Zhang XH, Liu YS, Li F et al (2012) 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. J Steroid Biochem Mol Biol 132:112–119
Xiang GD, Pu JH, Zhao LS, Sun HL, Hou J et al (2009) Association between plasma osteoprotegerin concentrations and urinary albumin excretion in Type 2 diabetes. Diabet Med 26:397–403
Yan W, Li X (2013) Impact of diabetes and its treatments on skeletal diseases. Front Med 7:81–90
Yuan LQ, Zhu JH, Wang HW, Liang QH, Xie H et al (2011) RANKL is a downstream mediator for insulin-induced osteoblastic differentiation of vascular smooth muscle cells. PLoS One 6:e29037
Zoidis E, Ghirlanda-Keller C, Schmid C (2011) Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem 348:33–42
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Nível Superior (CAPES), and Fundação Oswaldo Ramos (FOR).
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cunha, J.S., Ferreira, V.M., Maquigussa, E. et al. Effects of high glucose and high insulin concentrations on osteoblast function in vitro. Cell Tissue Res 358, 249–256 (2014). https://doi.org/10.1007/s00441-014-1913-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1913-x