Abstract
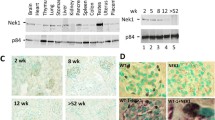
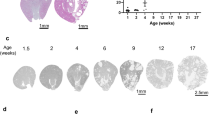
Mutations in the human NIMA (Never in Mitosis gene A)-related kinase 8 (Nek8) are associated with a rare form of the juvenile renal cystic disease, nephronophthisis type 9, and mutations in murine Nek8 cause renal cysts in jck mice. Cystogenesis involves dysfunctional ciliary signaling, and we have previously reported that Nek8 localizes to the primary cilium in mouse kidney epithelial cells. We now report that in developing mouse kidney, Nek8 is detected in the cilia of a subset of ureteric-bud-derived tubules at embryonic day (E)15.5. An increasing proportion of ureteric-bud-derived tubules express ciliary Nek8 until E18.5. Postnatal day 1 and 7 Nek8 is observed with equal frequency in both ureteric-bud and non-ureteric-bud-derived tubules. To investigate the cell biological consequences of kinase-deficient and jck mutant forms of Nek8, we transiently expressed green fluorescent protein (GFP)-tagged constructs in vitro. Mutations in the kinase and C-terminal domains of Nek8 adversely affected ciliary targeting but did not affect ciliogenesis or ciliary length. Consistent with these in vitro observations, kidneys from homozygous jck mice revealed reduced ciliary expression of Nek8 compared with kidneys from heterozygous (unaffected) mice. These data indicate that the ciliary localization of Nek8 in a subset of ureteric-bud-derived kidney tubules is essential for maintaining the integrity of those tubules in the mammalian kidney.






Similar content being viewed by others
References
Guay-Woodford LM (2006) Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol 21:1369–1376
Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6:928–940
Pazour GJ (2004) Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15:2528–2536
Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrant F (2007) Mutations in NIMA-related kinase NEK8 affects ciliary and centrosomal localization and may cause nephronophthisis in humans (in press)
Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR (2002) A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in the zebrafish. Development 129:5839–5846
Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O (2006) Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17:2821–2831
Mahjoub MR, Trapp ML, Quarmby LM (2005) NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16:3485–3489
Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O (2007) Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444:949–952
Parker JD, Bradley BA, Mooers AO, Quarmby LM (2007) Phylogenetic analysis of the neks reveals early diversification of ciliary-cell cycle kinases. PLoS ONE 2:e1076
O’Connell MJ, Krien MJ, Hunter T (2003) Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol 13:221–228
Davies JA, Bard JB (1998) The development of the kidney. Curr Top Dev Biol 39:245–301
Schedl A, Hastie ND (2000) Cross-talk in kidney development. Curr Opin Genet Dev 10:543–549
Dressler GR (2006) The cellular basis of kidney development. Annu Rev Cell Dev Biol 22:509–529
Mahjoub MR, Rasi MQ, Quarmby LM (2004) A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol Biol Cell 15:5172–5286
Quarmby LM, Mahjoub MR (2005) Caught Nek-ing: cilia and centrioles. J Cell Sci 118:5161–5169
Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE (2000) Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA 97:217–221
Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M (2006) Defective planar cell polarity in polycystic kidney disease. Nat Genet 38:21–23
Bissler JJ, Dixon BP (2005) A mechanistic approach to inherited polycystic kidney disease. Pediatr Nephrol 20:558–566
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151:709–718
Polgar K, Burrow CR, Hyink DP, Fernandez H, Thornton K, Li X, Gusella GL, Wilson PD (2005) Disruption of polycystin-1 function interferes with branching morphogenesis of the ureteric bud in developing mouse kidney. Dev Biol 286:16–30
Guillaume R, D’agati V, Daoust M, Trudel M (1999) Murine Pkd1 is a developmentally regulated gene from morula to adulthood: role in tissue condensation and patterning. Dev Dyn 214:337–348
Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R (2000) Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol 11:814–827
Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, Attie-Bitach T, Guicharnaud L, Devuyst O, Germino GG, Gubler MC (2002) Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol 160:973–983
Menezes LF, Cai Y, Nagasawa Y, Silva AM, Watkins ML, da Silva AM, Somolo S, Guay-Woodford LM, Germino GG, Onuchic LF (2004) Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66:1345–1355
Nürnberger J, Kavapurackal R, Zhang SJ, Saez AO, Heusch G, Philipp T, Pietruck F, Kribben A (2006) Differential tissue distribution of the Invs gene product inversin. Cell Tissue Res 323:147–155
Acknowledgements
We thank Lin Chen of the Rosenblum lab for invaluable assistance. We also thank Michel Leroux and his lab members for the use of their tissue culture facilities. MLT was supported by graduate fellowships from the Michael Smith Foundation for Health Research and the Natural Sciences and Engineering Research Council of Canada. Work in the Quarmby lab was supported by operating grants from the Kidney Foundation of Canada and by the Canadian Institutes of Health Research (MOP-37861). Work in the Rosenblum lab was supported by grants from the Canadian Institutes of Health Research and the Canada Research Chairs Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Condensing mesenchyme does not express NIMA (never in mitosis gene a)-related kinase 8 (Nek8). Paraffin-embedded sections of embryonic day (E)18.5 CD1/129 mouse kidneys stained with anti-Nek8 antibody, anti-neural-cell adhesion molecule (NCAM) antibody, and 4′,6-diamidino-2-phenylindole (DAPI) (blue). a Tubules in the cortex labeled with anti-NCAM (red) do not express luminal Nek8 (green). b NCAM-positive tubules (green) stained with anti-acetylated tubulin (red), indicating cilia (DOC 1.12 MB)
Fig. S2
In vitro ciliary measurements. Confluent inner medullary collecting duct (IMCD)-3 cells transfected with no DNA (mock), GFP alone, green fluorescent protein (GFP)-wild-type NIMA (never in mitosis gene a)-related kinase 8 (Nek8), GFP-K33M Nek8, and GFP-jck Nek8 were stained with anti-acetylated tubulin, and ciliary length was measured. Also shown is untransfected (untransf.) cells. The average of two trials is shown. Error bars = standard error of mean (DOC 145 KB)
Fig. S3
The jck renal morphology. Paraffin-embedded sections of postnatal day 7 jck heterozygous (+/jck) and homozygous (jck/jck) mouse kidneys from litter mates were stained with hematoxylin and eosin (DOC 6.00 MB)
Rights and permissions
About this article
Cite this article
Trapp, M.L., Galtseva, A., Manning, D.K. et al. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol 23, 377–387 (2008). https://doi.org/10.1007/s00467-007-0692-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0692-y