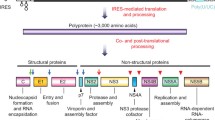
Abstract
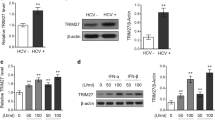
We previously demonstrated that hepatitis C virus (HCV) serine protease NS3-4A was unable to cleave TRIF (adaptor protein of Toll-like receptor 3), resulting in a lack of suppression of the TRIF-mediated pathway, whereas NS3-4A cleaved Cardif (adaptor protein of retinoic acid-inducible gene I or melanoma differentiation-associated gene-5), resulting in an interruption of the Cardif-mediated pathway in non-neoplastic human hepatocyte PH5CH8 cells. To elucidate these observations, we examined the cleavage potential of NS3-4A for TRIF in PH5CH8 cells, genome-length HCV RNA-replicating O cells, and HCV-infected cells, and we demonstrated that NS3-4A lacked the ability to cleave endogenous TRIF, regardless of HCV strains derived from patients with different stages of hepatic disease. Furthermore, we demonstrated that inflammatory cytokine production by NF-κB activation via the TRIF-mediated pathway also remained unsuppressed by NS3-4A. These results suggest that the inhibitory effects of NS3-4A on antiviral signaling pathways are limited to the Cardif-mediated pathway in human hepatocytes.






Similar content being viewed by others
References
Akagi T, Sasai K, Hanafusa H (2003) Refractory nature of normal human diploid fibroblasts with respect to oncogene-mediated transformation. Proc Natl Acad Sci USA 100:13567–13572
Alam SS, Nakamura T, Naganuma A, Nozaki A, Nouso K, Shimomura H, Kato N (2002) Hepatitis C virus quasispecies in cancerous and noncancerous hepatic lesions: the core protein-encoding region. Acta Med Okayama 56:141–147
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Benech P, Vigneron M, Peretz D, Revel M, Chebath J (1987) Interferon-responsive regulatory elements in the promoter of the human 2′, 5′-oligo(A) synthetase gene. Mol Cell Biol 7:4498–4504
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359–362
Dansako H, Ikeda M, Kato N (2007) Limited suppression of the interferon-β production by hepatitis C virus serine protease in cultured human hepatocytes. FEBS J 274:4161–4176
Dansako H, Ikeda M, Abe K, Mori K, Takemoto K, Ariumi Y, Kato N (2008) A new living cell-based assay system for monitoring genome-length hepatitis C virus RNA replication. Virus Res 137:72–79
Dansako H, Naganuma A, Nakamura T, Ikeda F, Nozaki A, Kato N (2003) Differential activation of interferon-inducible genes by hepatitis C virus core protein mediated by the interferon stimulated response element. Virus Res 97:17–30
Dansako H, Naka K, Ikeda M, Kato N (2005) Hepatitis C virus proteins exhibit conflicting effects on the interferon system in human hepatocyte cells. Biochem Biophys Res Commun 336:458–468
Ferreon JC, Ferreon AC, Lemon SM (2005) Molecular determinants of TRIF proteolysis mediated by the hepatitis C virus NS3/4A protease. J Biol Chem 280:20483–20492
Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K (1991) Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA 88:5547–5551
Hijikata M, Mizushima H, Tanji Y, Komada Y, Hirowatari Y, Akagi T, Kato N, Kimura K, Shimotohno K (1993) Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci USA 90:10773–10777
Honda K, Taniguchi T (2006) IRFs: master regulators of signaling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6:644–658
Ikeda M, Abe K, Dansako H, Nakamura T, Naka K, Kato N (2005) Efficient replication of a full-length hepatitis C virus genome, strain O, in cell culture, and development of a luciferase reporter system. Biochem Biophys Res Commun 329:1350–1359
Ikeda M, Kato N, Mizutani T, Sugiyama K, Tanaka K, Shimotohno K (1997) Analysis of the cell tropism of HCV by using in vitro HCV-infected human lymphocytes and hepatocytes. J Hepatol 27:445–454
Ikeda M, Sugiyama K, Mizutani T, Tanaka T, Tanaka K, Sekihara H, Shimotohno K, Kato N (1998) Human hepatocyte clonal cell lines that support persistent replication of hepatitis C virus. Virus Res 56:157–167
Jiang Z, Mak TW, Sen G, Li X (2004) Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc Natl Acad Sci USA 101:3533–3538
Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB (2002) mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA 99:637–642
Kato N (2001) Molecular virology of hepatitis C virus. Acta Med Okayama 55:133–159
Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkoshi S, Sugiyama T, Shimotohno K (1990) Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA 87:9524–9528
Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K (1993) Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol 67:3923–3930
Kishine H, Sugiyama K, Hijikata M, Kato N, Takahashi H, Noshi T, Nio Y, Hosaka M, Miyanari Y, Shimotohno K (2002) Subgenomic replicon derived from a cell line infected with the hepatitis C virus. Biochem Biophys Res Commun 293:993–999
Komoda Y, Hijikata M, Sato S, Asabe SI, Kimura K, Shimotohno K (1994) Substrate requirements of hepatitis C virus serine proteinase for intermolecular polypeptide cleavage in Escherichia coli. J Virol 68:7351–7357
Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE, Tegtmeier GE, Bonino F, Colombo WS, Lee WS, Kuo C, Berger K, Shuster JR, Overby LR, Bradley DW, Houghton M (1989) An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362–364
Kuroki M, Ariumi Y, Ikeda M, Dansako H, Wakita T, Kato N (2009) Arsenic trioxide inhibits hepatitis C virus RNA replication through modulation of the glutathione redox system and oxidative stress. J Virol 83:2338–2348
Lanford RE, Guerra B, Lee H, Averett DR, Pfeiffer B, Chavez D, Notvall L, Bigger C (2003) Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J Virol 77:1092–1104
Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM (2005) Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA 102:2992–2997
Li K, Chen Z, Kato N, Gale M Jr, Lemon SM (2005) Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem 280:16739–16747
Li XD, Sun L, Seth RB, Pineda G, Chen ZJ (2005) Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA 102:17717–17722
Meylan E, Curran J, Hofman K, Moradpour D, Binder M, Bartenschlager R, Tschopp J (2005) Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172
Naka K, Dansako H, Kobayashi N, Ikeda M, Kato N (2005) Hepatitis C virus NS5B delays cell cycle progression by inducing interferon-beta via Toll-like receptor 3 signaling pathway without replicating viral genomes. Virology 346:348–362
Ohkoshi S, Kojima H, Tawaraya H, Miyajima T, Kamimura T, Asakura H, Satoh A, Hirose S, Hijikata M, Kato N, Shimotohno K (1990) Prevalence of antibody against non-A, non-B hepatitis virus in Japanese patients with hepatocellular carcinoma. Jpn J Cancer Res 81:550–553
Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi Y, Watanabe S, Koi S, Onji M, Ohta Y, Choo QL, Houghton M, Kuo G (1990) Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA 87:6547–6549
Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S (2003) Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 171:4304–4310
RJr Sumpter, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, MJr Gale (2005) Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699
Tanaka T, Kato N, Cho MJ, Shimotohno K (1995) A novel sequence found at the 3′ terminus of hepatitis C virus genome. Biochem Biophys Res Commun 215:744–749
Thomas DL (2000) Hepatitis C epidemiology. Curr Top Microbiol Immunol 242:25–41
Ueta M, Hamuro J, Kiyono H, Kinoshita S (2005) Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun 331:285–294
Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB (2005) VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell 19:727–740
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737
Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, MJr Gale, Akira S, Yonehara S, Kato A, Fujita T (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 175:2851–2858
Wakita T, Pietschmann TT, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ (2005) Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796
Acknowledgments
We would like to thank T. Maeta, K. Takemoto, and T. Nakamura for their technical assistance. K. Naka and S. Ohkoshi are also thanked for their valuable input in this study. This work was supported by Grants-in-Aid for the Third-Term Comprehensive Ten-Year Strategy for Cancer Control, and by a Grant-in-Aid for Research on Hepatitis, both from the Ministry of Health, Labor, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1.
All NS3-4As derived from patients with different hepatic disease diagnoses prevent the activity of T-pIC-induced IFN-β gene promoter via a Cardif-mediated pathway. a Effects of 15 NS3-4As on the activity of IFN-β gene promoter. PH5CH8 cells transiently expressing NS3-4As from various HCV strains were subjected to T-pIC treatment. PH5CH8 cells transfected with pCX4bsr vector were used as a control (strain, -). Dual luciferase assay was performed described in Materials and Methods. Data are expressed as the means ± SD from three independent experiments, each of which was performed in triplicate. b All of the NS3-4As cleaved endogenous Cardif. PH5CH8 cells were transfected with the HA-Cardif-Flag and NS3-4A expression vectors. Production of HA-Cardif-Flag and NS3 was analyzed by immunoblotting using anti-Flag and anti-NS3 antibody, respectively. PH5CH8 cells transfected with the pCX4bsr and pCX4pur vectors were used as a control (-). β-actin was used as a control for the amount of protein loaded per lane. (TIFF 207 kb)
Rights and permissions
About this article
Cite this article
Dansako, H., Ikeda, M., Ariumi, Y. et al. Double-stranded RNA-induced interferon-beta and inflammatory cytokine production modulated by hepatitis C virus serine proteases derived from patients with hepatic diseases. Arch Virol 154, 801–810 (2009). https://doi.org/10.1007/s00705-009-0375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-009-0375-z