Abstract
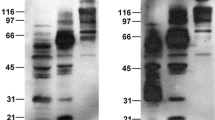
For the design of effective antiviral strategies, understanding the fundamental steps of the virus life cycle, including virus–host interactions, is essential. We performed a virus overlay protein binding assay followed by proteomics for identification of proteins from membrane fractions of A7 (Aedes aegypti) cells, C6/36 (Aedes albopictus) cells and the midgut brush border membrane fraction of Ae. aegypti mosquito that bind to dengue-2 virus. Actin, ATP synthase β subunit, HSc 70, orisis, prohibitin, tubulin β chain, and vav-1 were identified as dengue-2-virus-binding proteins. Our results suggest that dengue-2 virus exploits an array of housekeeping proteins for its entry in mosquito cells.



Similar content being viewed by others
References
Arispe N, De Maio A (2000) ATP and ADP modulate a cation channel formed by Hsc70 in acid phospholipid membranes. J Biol Chem 275:30839–30843
Black WC, Bennett KE, Escalante-Gorrochotegui N, Barillas-Mury CV, Fernandez-Salas I, LourdesMunoz M, Farfan-Ale JA, Olson KE, Beaty BJ (2002) Flavivirus susceptibility in Aedes aegypti. Arch Med Res 33:379–388
Cao-Lormeau VM (2009) Dengue viruses binding proteins from Aedes aegypti and Aedes polynesiensis salivary glands. Virol J 6:35
Chavez-Salinas S, Ceballos-Olvera I, Reyes-del Valle J, Medina F, del Angel RM (2008) Heat shock effect upon dengue virus replication into U937 cells. Virus Res 138:111–118
Chee H, AbuBakar S (2004) Identification of a 48 kDa tubulin or tubulin-like C6/36 mosquito cells protein that binds dengue virus 2 using mass spectrometry. Biochem Biophys Res Commun 320:11–17
Chu JJH, Ng ML (2002) Trafficking mechanism of West Nile (Sarafend) virus structural proteins. J Med Virol 67:127–136
Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S (2003) Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem 278:47853–47861
Heinz FX, Allison SL (2003) Flavivirus structure and membrane fusion. Adv Virus Res 59:63–97
Hong SS, Ng ML (1987) Involvement of microtubules in Kunjin virus replication. Arch Virol 97:115–121
Houk EJ, Arcus YM, Hardy JL (1986) Isolation and characterization of brush border fragments from mesenterons. Arch Insect Biochem Physiol 3:135–146
Hung SL, Lee PL, Chen HW, Chen LK, Kao CL, King CC (1999) Analysis of the steps involved in Dengue virus entry into host cells. Virology 257:156–167
Liu WJ, Qi YM, Zhao KN, Liu YH, Liu XS, Frazer IH (2001) Association of bovine papillomavirus type 1 with microtubules. Virology 282:237–244
Lourenco-de-Oliveira R, Vazeille M, de Filippis AM, Failloux AB (2004) Ae. aegypti in Brazil: genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Trans R Soc Trop Med Hyg 98:43–54
Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G (2001) Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online 3:19–25
McClung JK, Jupe ER, Liu XT, Dell’Orco RT (1995) Prohibitin: potential role in senescence, development and tumor suppression. Exp Gerontol 30:99–124
Mendoza YM, Salas-Benito JS, Lanz-Mendoza H, Hernandez-Martinez S, del Angel RM (2002) A putative receptor for dengue virus in mosquito tissues: localization of a 45-kDa glycoprotein. Am J Trop Med Hyg 67:76–84
Mercado-Curiel RF, WC Black IV, Lourdes Muñoz M (2008) A dengue receptor as possible genetic marker of vector competence in Aedes aegypti. BMC Microbiol 8:118
Mercado-Curiel RF, Esquinca-Avilés HA, Tovar R, Díaz-Badillo A, Camacho-Nuez M, de Muñoz ML (2006) The four serotypes of dengue recognize the same putative receptors in Aedes aegypti midgut and Ae. albopictus cells. BMC Microbiol 6:85
Mishra S, Moulik S, Murphy LJ (2007) Prohibitin binds to C3 and enhances complement activation. Mol Immunol 44:1897–1902
Mishra S, Murphy LC, Murphy LJ (2006) The Prohibitins: emerging roles in diverse functions. J Cell Mol Med 10:353–363
Modis Y, Ogata S, Clements D, Harrison SC (2003) A ligand binding pocket in Dengue virus envelop glycoprotein. Proc Natl Acad Sci USA 100:6986–6991
Moyer SA, Baker SC, Lessard JL (1986) Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci USA 83:5405–5409
Ng ML, Hong SS (1989) Flavivirus infection: essential ultrastructural changes and association of Kunjin virus NS3 protein with microtubules. Arch Virol 106:103–120
Padwad YS, Mishra KP, Jain M, Chanda S, Karan D, Ganju L (2009) RNA interference mediated silencing of Hsp60 gene in human monocytic myeloma cell line U937 revealed decreased dengue virus multiplication. Immunobiology 214:422–429
Ren J, Ding T, Zhang W, Song J, Ma W (2007) Does Japanese encephalitis virus share the same cellular receptor with other mosquito-borne flaviviruses on the C6/36 mosquito cells? Virol J 4:83
Rey FA (2003) Dengue virus envelope glycoprotein structure: new insight into its interactions during viral entry. Proc Natl Acad Sci USA 100:6899–6901
Reyes Del Valle J, Chávez-Salinas S, Medina F, del Angel RM (2005) Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol 79:4557–4567
Salas-Benito J, Valle JR-D, Salas-Benito M, Ceballos-Olvera I, Mosso C, Del Angel RM (2007) Evidence that the 45-kD glycoprotein, part of a putative dengue virus receptor complex in the mosquito cell line C6/36, is a heat-shock-related protein. Am J Trop Med Hyg 77:283–290
Salas-Benito JS, del Angel RM (1997) Identification of two surface proteins from C6/36 cells that bind dengue type 4 virus. J Virol 71:7246–7252
Sharma A, Qadri A (2004) Vi polysaccharise of Salmonella typhi target the prohibitin family of molecules in intestinal epithelial cells and suppress early inflammatory responses. Proc Natl Acad Sci USA 101:17492–17497
Smith GA, Enquist LW (2002) Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu Rev Cell Dev Biol 18:135–161
Suomalainen M, Nakano MY, Boucke K, Keller S, Stidwill RP, Greber UF (1999) Microtubule-dependent minus and plus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol 144:657–672
Talavera D, Castillo AM, Dominguez MC, Gutierrez AE, Meza I (2004) IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J Gen Virol 85:1801–1813
Upanan S, Kuadkitkan A, Smith DR (2008) Identification of dengue virus binding proteins using affinity chromatography. J Virol Methods 151:325–328
Villas-Bôas CSA, Conceição TM, Ramírez J, Santoro ABM, Da Poian AT, Montero-Lomelí M (2009) Dengue virus-induced regulation of the host cell translational machinery. Braz J Med Biol Res 42(11):1020–1026
Wang S, Zhang B, Faller DV (1999) Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol Cell Biol 19:7447–7460
Waterboer T, Rahaus M, Wolff MH (2002) Varicellazoster virus (VZV) mediates a delayed host shutoff independent of open reading frame (ORF) 17 expression. Virus Genes 24:49–56
Wolfersberger M, Liithy P, Maurer A, Parenti P, Sacchi VF, Giordana B, Hanozel GM (1987) Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp Biochem Physiol 86:301–308
Xu A, Bellamy AR, Taylor JA (2000) Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules. EMBO J 19:6465–6474
Acknowledgments
The authors acknowledge valuable suggestions given by Deepti Deobagkar. We thank A.C. Mishra, National Institute of Virology, Pune, for the facilities and encouragement and Dipankar Chaterjee, Indian Institute of Science, Bangalore, for the MALDI TOF/TOF facility. This research was supported by UGC-CAS and ICMR grants of DND. MSP is a CSIR Senior Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paingankar, M.S., Gokhale, M.D. & Deobagkar, D.N. Dengue-2-virus-interacting polypeptides involved in mosquito cell infection. Arch Virol 155, 1453–1461 (2010). https://doi.org/10.1007/s00705-010-0728-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-010-0728-7