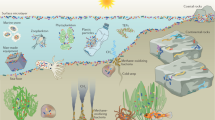
Abstract
In the marine environment, biofilms on submerged surfaces can promote or discourage the settlement of invertebrate larvae and macroalgal spores. The settlement-mediating effects of biofilms are believed to involve a variety of biofilm attributes including surface chemistry, micro-topography, and a wide range of microbial products from small-molecule metabolites to high-molecular weight extracellular polymers. The settled organisms in turn can modify microbial species composition of biofilms and thus change the biofilm properties and dynamics. A better understanding of biofilm dynamics and chemical signals released and/or stored by biofilms will facilitate the development of antifouling and mariculture technologies. This review provides a brief account of 1) existing knowledge of marine biofilms that are relevant to settlement mediation, 2) biotechnological application of biofilms with respect to developing non-toxic antifouling technologies and improving the operation of aquaculture facilities, and 3) challenges and future directions for advancing our understanding of settlement-mediating functions of biofilms and for applying this knowledge to real-life situations.
Similar content being viewed by others
References
Acuňa N, Ortega-Morales BO, Valadez-Gonzalez A (2006) Biofilm colonization dynamics and its influence on the corrosion resistance of austenitic UNS S31603 stainless steel exposed to Gulf of Mexico seawater Mar Biotech (in press)
Allison DG (2003) The biofilm matrix Biofouling 19, 139–150
Armstrong E, Yan LM, Boyd, KG, Wright PC, Burgess JG (2001) The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461, 37–40
Baloun AJ, Morse DE (1984) Ionic control of metamorphosis in larval Haliotis rufescens (Gastropoda). Biol Bull 167, 124–138
Baxter GT, Morse DE (1992) Cilia from abalone larvae contain a receptor-dependent G protein transduction system similar to that in mammals. Biol Bull 183, 147–154
Beckmann M, Harder T, Qian PY (1999) Induction of larval attachment and metamorphosis in the serpulid polychaete Hydroides elegans by dissolved free amino acids: mode of action in laboratory bioassays. Mar Ecol Prog Ser 190, 167–178
Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2006) Marine natural products. Natural Prod Rep 23, 26–78
Borenstein SB (1994) Microbiologically Influenced Corrosion Handbook (New York: Industrial Press)
Braithwaite RA, McEvou LA (2005) Marine biofouling on fish farms and its remediation. Adv Mar Biol 47, 15–52
Callow ME, Callow JA (2000) Substratum location and zoospore behavior in the fouling alga Enteromorpha. Biofouling 15, 49–56
Carpizo-Ituarte EJ, Hadfield MG (2003) Transcription and translation inhibitors permit metamorphosis up to radiole formation in the serpulid polychaete Hydroides elegans Haswell. Biol Bull 204, 114–125
Charaklis WG, Cooksey B (1983) Biofilm and microbial fouling. Adv Appl Microbiol 29, 93–148
Chia F-S, Koss R (1982) Fine structure of the larval rhinophores of the nudibranch, Rostanga pulchra, with emphasis on the sensory receptor cells. Cell Tissue Res 225, 235–248
Chia F-S, Koss R (1984) Fine structure of the cephalic sensory organ in the larva of the nudibranch Rostanga pukhra (Mollusca, Opisthobranchia, Nudibranchia). Zoomorphology 104, 131–139
Chia F-S, Koss R (1989) The fine structure of the newly discovered propodial ganglia of the veliger larva of the nudibranch, Onchidoris bilamellate. Cell Tissue Res 256, 17–26
Chia F-S, Koss R, Stevens S, Goldberg JI (1992) Isolation of neurons of a nudibranch veliger. Biol Bull 182, 66–76
Clare AS (1998) Towards non-toxic antifouling. J Mar Biotechnol 6, 3–6
Cooksey B, Cooksey KE, Miller CA, Paul JP, Webster D, Rubin RW (1984) The attachment of microfouling diatoms. In: Marine Biodeterioration: An Interdisciplinary Study, Costlow JD, Tipper RD, eds. (Annapolis, MD: US Naval Institute Press)
Cooksey KE, Wiggleworth-Cooksey B (1995) Adhesion of bacteria and diatoms to surfaces in the sea-a review. Aquat Microb Ecol 9, 87–96
Dahms H-U, Dobretsov S, Qian P-Y (2004) The effect of bacterial and diatom biofilms on the settlement of the bryozoan Bugula neritina. J Exp Mar Biol Ecol 313, 191–209
Dahms H-U, Qian P-Y (2005) Exposure of biofilms to meiofaunal copepods affects the larval settlement of Hydroides elegans (Polychaeta). Mar Ecol Prog Ser 297, 203–214
Dang H, Lovell CR (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl Environ Microbiol 66, 467–475
Daume S, Brand-Gardner S, Woelkerling WJ (1999) Preferential settlement of abalone larvae: diatom films vs non-geniculate coralline red algae. Aquaculture 174, 243–254
Decho AW, Browne KA, Zimmer-Faust RK (1998) Chemical cues: Why basic peptides are signal molecules in marine environments. Limnol Oceanogr 43, 1410–1417
Degnan BM, Morse DE (1995) Developmental and morphogenetic gene regulation in Haliotis rufescens larvae at metamorphosis. Am Zool 35, 391–398
Demain AL, Davies JE, eds. (1999) Manual of Industrial Microbiology and Biotechnology. (Washington, DC: ASM Press)
De Nys R, Steinberg PD, Willemson P, Dworjanyn SA, Gabelish CL, King RJ (1995) Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling 8, 259–271
Dobretsov S, Qian P -Y (2002) Effect of bacteria associated with the green alga Ulva reticulata on marine micro- and macrofouling. Biofouling 18(3), 217–228
Dobretsov S, Qian P-Y (2004) The role of epibotic bacteria from the surface of the soft coral Dendronephthya sp in the inhibition of larval settlement. J Exp Mar Biol Ecol 299, 35–50
Dobretsov S, Qian P-Y (2006) Facilitation and inhibition of larval attachment of the bryozoan Bugula neritina in association with mono-species and multi-species biofilms. J Exp Mar Biol Ecol 333, 263–274
Dobretsov S, Dahms H-U, Qian P -Y (2006) Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 22, 43–54
Dreanno C, Kirby RR, Clare AS (In press) Locating the barnacle settlement pheromone: spatial and ontogenetic expression of the settlement-inducing protein complex of Balanus amphitrite. Proc R Soc B (Doi: 101098/rspb20063649online)
Dreanno C, Matsumura K, Dohmae N, Takio K, Hirota H, Kirby RR, Clare AS (2006) An a2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus Amphitrite. PNAS 103, 14396–14401
Dworjanin SA, De Nys R, Steinber P (2006) Chemically mediated antifouling in the red alga Delisea pulchra. Mar Ecol Prog Ser 318, 153–163
Egan S, James S, Holmstrøm C, Kjelleberg S (2001) Inhibition of algal spore germination by the marine bacterium Pseudoalteromonas tunicata. FEMS Microbiol Ecol 5, 67–73
Eilers H, Pernthaler J, Gloeckner FO, Amann R (2000) Culturability and in situ abundance of pelagic bacteria in the North Sea. Appl Environ Microbiol 66, 3044–3051
Eri R, Arnold JM, Hinman VF, Green KM, Jones MK, Degan BM, Lavin MF (1999) Hemps, a novel EGF-like protein, plays a central role in ascidian metamorphosis. Development 126, 5809–5818
Evans LN, Hoagland KD (1986) Algal Biofouling. (Amsterdam: Elsevier)
Fusetani N (2004) Biofouling and antifouling. Nat Prod Rep 21, 94–104
Gil-Turnes MS, Fenical W (1992) Embryos of Homarus americanus are protected by epibiotic bacteria. Biol Bull 182, 105–108
Gil-Turnes MS, Hay ME, Fenical W (1989) Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246, 116–118
Golz WJ, Rusch KA, Malone RF (1999) Modeling the major limitations on nitrification in floating-bead filters. Aquacult Eng 20, 43–62
Greenberg EP (2003) Bacterial communication and group behavior. J Clin Invest 112, 1288–1290
Hadfield MG, Paul VJ (2001) Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: Marine Chemical Ecology, McClintock JB, Baker W, eds. (Boca Raton, FL: CRC Press) pp 431–461
Hadfield MG, Meleshkevitch E, Boudko D (2000) The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol Bull 198, 67–76
Harder T, Lam C, Qian, P-Y (2002a) Induction of larval settlement in the polychaete Hydroides elegans by marine biofilms: an investigation of monospecific diatom films as settlement cues. Mar Ecol Prog Ser 229, 105–112
Harder T, Lau SCK, Dahms H-U, Qian P-Y (2002b) Isolation of bacterial metabolites as natural inducers for larval settlement in the marine polychaete Hydroides elegans (Haswell). J Chem Ecol 28(10), 2029–2043
Holmstrøm C, Kjelleberg S (1999) Marine Pseudoalteromonas species are associated with higher organisms and produce active extracellular compounds. FEMS Microbiol Ecol 30, 285–293
Holmstrøm C, Rittschof D, Kjelleberg S (1992) Inhibition of settlement by larvae of Balanus amphitrite and Cliona intestinalis by a surface-colonizing marine bacterium. Appl Environ Microb 58, 2111–2115
Holmstrøm C, James S, Egan S, Kjelleberg S (1996) Inhibition of common fouling organisms by marine bacterial isolates with special reference to the role of pigmented bacteria. Biofouling 10, 251–259
Holmstrøm C, Egan S, Francks A, McCloy S, Kjelleberg S (2002) Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol Ecol 41, 47–58
Huang S, Hadfield MG (2003) Composition and density of bacterial biofilms affect metamorphosis of the polychaete Hydroides elegans. Mar Ecol Prog Ser 260, 161–172
Hung OS, Thiyagarajan V, Wu RSS, Qian P-Y (2005a) Effects of ultraviolet radiation on films and subsequent settlement of Hydroides elegans. Mar Ecol Prog Ser 304, 155–166
Hung OS, Gosselin LA, Thiyagarajan V, Wu RSS, Qian P-Y (2005b) Do effects of ultraviolet radiation on microbial films have indirect effects on larval attachment of the barnacle Balanus amphitrite. J Exp Mar Biol Ecol 323, 16–26
Ista LK, Fan HY, Baca O, Lopez GP (1996) Attachment of bacteria to model solid surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microbiol Lett 142, 59–63
Jin T, Qian PY (2004) Effect of mono-amino acids on larval metamorphosis of the polychaete Hydroides elegans. Mar Ecol Prog Ser 267, 223–232
Jin T, Qian PY (2005) Amino acid exposure modulates the bioactivity of biofilms for larval settlement of Hydroides elegans by altering bacterial community components. Mar Ecol Prog Ser 297, 169–179
Joint I, Callow ME, Callow JA, Clarke KR (2002a) The attachment of Enteromorpha zoospores to a bacterial biofilm assemblage. Biofouling 16, 151–158
Joint I, Tait K, Callow ME, Callow JA, Milton D, Williams P, Camara M (2002b) Cell-to-cell communication across the prokaryote-eukaryote boundary. Science 298, 1207–1207
Kang KH, Kim BH, Kim JM (2004) Induction of larval settlement and metamorphosis of the abalone, Haliotis discus hannai larvae using bromomethane and potassium chloride. Aquaculture 230, 249–259
Keough MJ, Raimondi PT (1996) Responses of settling invertebrate larvae to bioorganic films: effects of large-scale variation in films. J Exp Mar Biol Ecol 207(1–2), 59–78
Kirchman D, Graham D, Reish D, Mitchell R (1982) Lectins may mediate in the settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirorbidae). Mar Biol Lett 3, 201–222
Kjelleberg S, Albertson N, Flärdh K, Holmquist L, Jouper-Jaan A, Marouga R, Östling J, Svenblad B, Weichart D (1993) How do non-differentiating bacteria adapt to starvation? Anton v Leeuwen 63, 333–341
Kon-ya K, Shimidzu N, Otaki N, Yokoyama A, Adachi K, Miki W (1995) Inhibitory effect of bacterial ubiquinones on the settling of barnacle, Balanus amphitrite. Experientia 51, 153–155
Lam C, Harder T, Qian P-Y (2003) Induction of larval settlement in the polychaete Hydroides elegans by surface-associated settlement cues of marine benthic diatoms. Mar Ecol Prog Ser 263, 83–92
Lam C, Harder T, Qian P -Y (2005a) Induction of larval settlement in the polychaete Hydroides elegans by extracellular polymers of benthic diatoms. Mar Ecol Prog Ser 286, 145–154
Lam C, Harder T, Qian P-Y (2005b) Different growth conditions of benthic diatoms affect quality and quantity of extracellular polymeric larval settlement cues. Mar Ecol Prog Ser 294, 109–116
Lappin-Scott HM, Costerton JM (1989) Bacterial biofilms and surface fouling. Biofouling 1, 323–342
Lau SCK, Mak KK, Chen F, Qian P-Y (2002) Bioactivity of bacterial strains from marine biofilms in Hong Kong waters for the induction of larval settlement in the marine polychaete Hydroides elegans. Mar Ecol Prog Ser 226, 301–310
Lau SCK, Harder T, Qian P-Y (2003a) Induction of larval settlement in the serpulid polychaete Hydroides elegans (Haswell): role of bacterial extracellular polymers. Biofouling 19, 197–204
Lau SCK, Thiyagarajan V, Qian P-Y (2003b) The bioactivity of bacterial isolates in Hong Kong waters for the inhibition of barnacle (Balanus amphitrite Darwin) settlement. J Exp Mar Biol Ecol 282, 43–60
Lau SCK, Thiyagarajan V, Cheung SCK, Qian P-Y (2005) Roles of bacterial community composition in biofilms as a mediator for larval settlement of three marine invertebrates. Aquat Microb Ecol 38, 41–51
Levin LA (2006) Recent progress in understanding larval dispersal: new directions and digressions. Integr Comp Biol 46, 282–297
Li X, Dobretsov S, Xu Y, Xiao X, Qian P-Y (2006) Antifouling diketopiperazines produced by a deep-sea bacterium Streptomyces fungicidicus. Biofouling (in press)
Little BJ, Zsolnay ZA (1985) Chemical fingerprinting of adsorbed organic materials on metal surfaces. J Colloid Interface Sci 104, 79–86
Maki JS (1999) The influence of Marine microbes on biofouling. In: Recent Advances in Marine Biotechnology, Vol. 3: Biofilms, Bioadhesion, Corrosion, and Biofouling. Fingerman M, Nagabhushanam R, Thompson F, eds. (Enfield, NH: Science Publishers) pp 147–171
Maki JS (2002) Biofouling in the marine environment. In: Encyclopedia of Environmental Microbiology. Bitton G, ed. (New York: John Wiley & Sons) pp 610–619
Maki JS, Rittschof D, Costlow JD, Mitchell R (1988) Inhibition of attachment of larval barnacles, Balanus amphitrite, by bacterial surface films. Mar Biol 97, 199–206
Maki JS, Ding L, Stokes J, Kavouras JH, Rittschof D (2000) Substratum/ bacterial interactions and larval attachment: films and exopolysaccharides of Halomonas marina (ATCC 25374) and their effect on barnacle cyprid larvae, Balanus amphitrite Darwin. Biofouling 16, 159–170
Marshall PD, Bowden GHW (2000) Microbial community interaction in biofilms. In Community Structure and Cooperation in Biofilms. Society for General Microbiology Symposia 59, Allison DG, Gilbert P, Lappin-Scott HM, Wilson M, eds. (Cambridge) pp 167–198
Mitchell R, Chet I (1975) Bacterial attack of corals in polluted seawater. Microb Ecol 2, 227–233
Mitchell R, Maki JS (1988) Microbial surface films and their influence on larval settlement and metamorphosis in the marine environment. In: Marine Biodeterioration: Advanced Techniques Applicable to the Indian Ocean. Thompson M-F, Sarojini R, Nagabushanam R, eds. (New Delhi: Oxford & IBH) pp 489–497
Morse ANC, Morse DE (1984) Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J Exp Mar Biol Ecol 74, 191–215
Morse ANC, Froyd CA, Morse DE (1984) Molecules from cyanobacteria and red algae that induce larval settlement and metamorphosis in the mollusk Haliotis rufescens. Mar Biol 81, 293–298
Olguin-Uribe G, Abou-Mansour E, Boulander A, Debard H, Francisco C, Combaut G (1997) 6-Bromoindole-3-carbaldehyde from an Acinetobacter sp bacterium associated with the ascidian Stomoza murrayi. J Chem Ecol 23, 2507–2521
Paerl HW, Pinckney JL (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol 31, 225–247
Parsek MR, Greenberg EP (1999) Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol 310, 43–55
Patel P, Callow ME, Joint I, Callow JA (2003) Specificity in larval settlement modifying response of bacterial biofilms towards zoospores of the marine alga. Enteromorpha Environ Microb 5, 338–349
Paul VJ, Puglisi MP, Ritson-Williams R (2006) Marine chemical ecology. Nat Prod Rep 23, 153–180
Qian P-Y (1999) Larval settlement of polychaetes. Hydrobiologia 402, 239–253
Qian P-Y, Thiyagarajan V, Lau SCK, Cheung SCK (2003) Bacterial community profile in biofilm and attachment of the acorn barnacle Balanus amphitrite. Aquat Microb Ecol 33, 225–237
Railkin AI (2004) Marine Biofouling: Colonization Processes and Defenses. CRC Press
Rice SA, Givskov M, Steinberg P, Kjelleberg S (1999) Bacterial signals and antagonists: the interaction between bacteria and higher organisms. J Mol Microbiol Biotechnol 1, 23–31
Riquelme C, Hayashida G, Araya R, Uchida A, Satomi M, Ishida Y (1996) Isolation of a native bacterial strain from the scallop Agropecten purpuratus with inhibitory effects against pathogenic Vibrios. J Shellfish Res 15, 369–374
Rittschof D (2000) Natural product antifoulants: one perspective on the challenges related to coatings development. Biofouling 15, 119–125
Schneider RP, Marshall KC (1994) Retention of the Gram negative marine bacterium SW8 on surfaces—effects of microbial physiology, substratum nature and conditioning films. Colloids Surf B Biointerfaces 2, 387–396
Seaver EC, Kaneshige LM (2006) Expression of ‘segmentation’; genes during larval and juvenile development in the polychaetes Capitella sp I and H elegans. Dev Biol 289, 179–194
Seaver EC, Thamm K, Hill S (2005) Growth patterns during segment formation in the two annelids Capitella sp I and Hydroides elegans: comparisons at distinct life history stages. Evol Dev 7, 312–326
Slattery M (1992) Larval settlement and juvenile survival in the red abalone (Haliotis rufescens): an examination of inductive cues and substrate selection. Aquaculture 102, 143–153
Steinberg PD, De Nys R, Kjelleberg S (2002) Chemical cues for surface colonization. J Chem Ecol 28, 1935–1951
Szewzyk U, Holmstrøm C, Wrangstadh M, Samuelsson MO, Maki JS, Kjelleberg S (1991) Relevance of the exopolysaccharide of marine Pseudomonas sp strain S9 for the attachment of Ciona intestinalis larvae. Mar Ecol Prog Ser 75, 259–265
Tait K, Joint I, Daykin M, Milton DL, Williams P, Camara M (2005) Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ Microbiol 7, 229–240
Tamburri MN, Zimmer-Faust RK, Tamplin LL (1992) Natural sources and properties of chemical inducers mediating settlement of oyster larvae: a re-examination. Biol Bull 183, 327–338
Tamburri MN, Finelli CM, Wethey DS, Zimmer-Faust RK (1996) Chemical induction of larval settlement. Biol Bull 191, 367–373
Thiyagarajan V, Hung OS, Chiu JMY, Wu RSS, Qian P-Y (2005) Growth and survival of juvenile barnacle Balanus amphitrite: interactive effects of cyprid energy reserve and habitat. Mar Ecol Prog Ser 299, 229–237
Todd CD, Keough MJ (1994) Larval settlement in hard substratum epifaunal assemblages: a manipulative field study of the effects of substratum filming and the presence of incumbents. J Exp Mar Biol Ecol 181, 159–187
Trapido-Rosenthal HG, Morse DE (1986) Availability of chemosensory receptors is down-regulated by habituation of larvae to a morphogenetic signal. PNAS 83, 7658–7662
Turner EJ, Zimmer-Faust RK, Palmer MA, Luckenbach M (1994) Settlement of oyster (Crassostrea virginica) larvae: effects of water flow and a water-soluble chemical cue. Limnol Oceanogr 39, 1579–1593
Wahl M (1997) Living attached: aufwuchs, fouling, epibiosis. In: Fouling Organisms of the Indian Ocean: Biology and Control Technology. Nagabushanam R, Thompson M, eds. (New Delhi: Oxford & IBH) pp 31–84
Weiner R, Sledjeski D, Quintero E, Coon S, Walch M (1993) Periphytic bacteria cue oyster larvae to set on fertile benthic biofil larvae by microbial biofilm cues. Biofouling 12, 81–93
Wheeler GL, Tait K, Taylor A, Brownlee C, Joint I (2006) Acyl-homoserine lactones modulate the settlement of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism. Plant Cell Environ 29, 608–616
Whelan A, Regan F (2006) Antifouling strategies for marine and riverine sensors. J Environ Monit 8, 880–886
Wimpenny J (1996) Ecological determinants of biofilm formation. Biofouling 10, 43–63
Yan LM, Boyd KG, Adams DR, Burgess JG (2003) Biofilm-specific cross-species induction of antimicrobial compounds in bacilli. Appl Environ Microbiol 69, 3719–3727
Yebra DM, Kiil S, Dam-Johansen K (2004) Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog Org Coatings 50, 75–104
Zhao B, Zhang S, Qian, P -Y (2003) Larval settlement of the silver- or goldlip pearl oyster Pinctada maxima (Jameson) in response to natural biofilms and chemical cues. Aquaculture 220, 883–901
Zimmer-Faust RK, Tamburri MN (1994) Chemical identity and ecological implications of a waterborne, larval settlement cue. Limnol Oceanogr 39, 1075–1087
Zobell CE, Allen EC (1935) The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol 29(3), 239–251
Acknowledgments
We thank Dr. J. Pechenik for his comments on the manuscript. This work was supported by research grants (CAS-CF03/04, HKUST 6402/05M, COMRRDA04/05.SC01, CA04/05.SC01) to P.Y. Qian.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, PY., Lau, S.C.K., Dahms, HU. et al. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar Biotechnol 9, 399–410 (2007). https://doi.org/10.1007/s10126-007-9001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9001-9