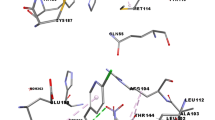
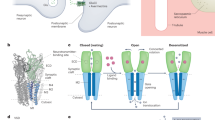
Abstract
Nicotinic acetylcholine receptors (nAChRs) are major excitatory neurotransmitter receptors in both vertebrates and invertebrates. In insects, nAChRs are the target site for several naturally occurring and synthetic compounds that exhibit potent insecticidal activity. Several compounds isolated from plants are potent agonists or antagonists of nAChRs, suggesting that these may have evolved as a defence mechanism against insects and other herbivores. Nicotine, isolated from the tobacco plant, has insecticidal activity and has been used extensively as a commercial insecticide. Spinosad, a naturally occurring mixture of two macrocyclic lactones isolated from the microorganism Saccharopolyspora spinosa, acts upon nAChRs and has been developed as a commercial insecticide. Since the early 1990s, one of the most widely used and rapidly growing classes of insecticides has been the neonicotinoids. Neonicotinoid insecticides are potent selective agonists of insect nAChRs and are used extensively in both crop protection and animal health applications. As with other classes of insecticides, there is growing evidence for the evolution of resistance to insecticides that act on nAChRs.




Similar content being viewed by others
References
Bai D, Lummis SCR, Leicht W, Breer H, Sattelle DB (1991) Actions of imidacloprid and a related nitromethylene on cholinergic receptors of an identified insect motor neurone. Pestic Sci 33:197–204
Bargmann CI (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282:2028–2033
Bass C, Lansdell SJ, Millar NS, Schroeder I, Turberg A, Field LM, Williamson MS (2006) Molecular characterisation of nicotinic acetylcholine receptor subunits from the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). Insect Biochem Mol Biol 36:86–96
Baumann A, Jonas P, Gundelfinger ED (1990) Sequence of Dα2, a novel α-like subunit of Drosophila nicotinic acetylcholine receptors. Nucleic Acids Res 18:3640
Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, Gundelfinger ED (1994) Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila α subunits. Eur J Neurosci 6:869–875
Bloomquist JR (1996) Ion channels as targets for insecticides. Ann Rev Entomol 41:163–190
Bockaert J (2001) G protein-coupled receptors. In: Encyclopedia of life sciences. Wiley, Chichester. http://www.els.net/, doi:10.1038/npg.els.0000118
Bossy B, Ballivet M, Spierer P (1988) Conservation of neural nicotinic acetylcholine receptors from Drosophila to vertebrate central nervous systems. EMBO J 7:611–618
Breer H, Sattelle DB (1987) Molecular properties and functions of insect acetylcholine receptors. J Insect Physiol 33:771–790
Buckingham SD, Balk ML, Lumis SCR, Jewess P, Sattelle DB (1995) Actions of nitromethylenes on an α-bungarotoxin-sensitive neuronal nicotinic acetylcholine receptor. Neuropharmacology 34:591–597
Buckingham SD, Biggin PC, Sattelle BM, Brown LA, Sattelle DB (2005) Insect GABA receptors: splicing, editing, and targeting by antiparasitics and insecticides. Mol Pharmacol 68:942–951
Cahill M, Denholm I (1999) Managing resistance to the chloronicotinyl insecticides—rhetoric or reality? In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor, Springer, Tokyo, pp 253–270
Cahill M, Gorman K, Day S, Denholm I, Elbert A, Nauen R (1996) Baseline determination and detection of resistance to imidacloprid in Bemisia tabaci (Homoptera: Aleyrodidae). Bull Entomol Res 86:343–349
Casida JE, Quistad GB (1998) Golden age of insecticide research: past, present and future. Ann Rev Entomol 43:1–16
Chouinard SW, Geng C, Orr N, G. G, Mitchell J, Cook K, Stilwell G (2006) Insecticide mode-of-action: gaining insight through model organism genetics. In: 11th IUPAC International Congress of Pesticide Chemistry Abstracts, Kobe, Japan, p 42
Clementi F, Fornasari D, Gotti C (2000) Neuronal nicotinic receptors, important new players in brain function. Eur J Pharmacol 393:3–10
Copping LG, Menn JJ (2000) Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci 56:651–676
Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M (1990) A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5:847–856
Dale HH (1914) The action of certain esters and ethers of choline and their relation to muscarine. J Pharmacol Exp Ther 6:147–190
Déglise P, Grünewald B, Gauthier M (2002) The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci Lett 321:13–16
Denholm I, Horowitz AR, Cahill M, Ishaaya I (1998) Management of resistance to novel insecticides. In: Ishaaya I, Degheele D (eds) Insecticides with novel modes of action: mechanisms and application. Springer, Berlin, pp 260–282
Devine GJ, Harling ZK, Scarr AW, Devonshire AL (1996) Lethal and sublethal effects of imidacloprid on nicotine-tolerant Myzus nicotinanae and Myzus persicae. Pestic Sci 48:57–62
Dhingra S (1994) Development of resistance in the bean aphid, Aphis craccivora Koch to various insecticides used for nearly a quarter century. J Entomol Res 18:105–108
Dudai Y (1978) Properties of an α-bungarotoxin binding cholinergic nicotinic receptor from Drosophila melanogaster. Biochem Biophys Acta 539:505–517
Eastham HM, Lind RJ, Eastlake JL, Clarke BS, Towner P, Reynolds SE, Wolstenholme AJ, Wonnacott S (1998) Characterization of a nicotinic acetylcholine receptor from the insect Manduca sexta. Eur J Neurosci 10:879–889
Elbert A, Nauen R, Leicht W (1998) Imidacloprid, a novel chloronicotinyl insecticide: biological activity and agricultural importance. In: Ishaaya I, Degheele D (eds) Insecticides with novel modes of action: mechanisms and applications. Springer, Berlin, pp 50–73
ffrench-Constant RH (1999) Target site mediated insecticide resistance: what questions remain? Insect Biochem Mol Biol 29:397–403
Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, Fire A, Sulston JE, Barnard EA, Sattelle DB, Lewis JA (1997) Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci 17:5843–5857
Foster SP, Denholm I, Thompson R (2003) Variation in response to neonicotinoid insecticides in peach-potato aphids, Myzus persicae (Hemiptera: Aphididae). Pest Manag Sci 59:166–173
Gepner JI, Hall LM, Sattelle DB (1978) Insect acetylcholine receptors as a site of insecticide action. Nature 276:188–190
Grauso M, Reenan RA, Culetto E, Sattelle DB (2002) Novel putative nicotinic acetylcholine receptor subunit genes, Dα5, Dα6 and Dα7, in Drosophila melanogaster identify a new and highly conserved traget of adenosine deaminase acting on RNA-mediated A-to-I pre-mRNA editing. Genetics 160:1519–1533
Gundelfinger ED, Schulz R (2000) Insect nicotinic acetylcholine receptors: genes, structure, physiological and pharmacological properties. In: Clementi F, Fornasari D, Gotti C (eds) Handbook of experimental pharmacology, vol 144. Neuronal nicotinic receptors. Springer, Berlin, pp 497–521
Gunning R (2002) Heliothis insecticides resistance continues to climb. Aust Cottongrower 23:71
Hama H (1986) Resistance spectrum to various insecticides in the diamondback moth, Plutella xylostella LINNÉ (Lepidoptera: Yponomeutidae). Jap J Appl Entomol Zool 30:277–284
Hermans-Borgmeyer I, Zopf D, Ryseck R-P, Hovemann B, Betz H, Gundelfinger ED (1986) Primary structure of a developmentally regulated nicotinic acetylcholine receptor protein from Drosophila. EMBO J 5:1503–1508
Hermsen B, Stetzer E, Thees R, Heiermann R, Schrattenholz A, Ebbinghaus U, Kretschmer A, Methfessel C, Reinhardt S, Maelicke A (1998) Neuronal nicotinic receptors in the Locust Locusta migratoria: cloning and expression. J Biol Chem 273:18394–18404
Herron GA, James TM (2005) Monitoring insecticide resistance in Australian Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) detects fipronil and spinosad resistance. Aust J Entomol 44:299–303
Huang Y, Williamson MS, Devonshire AL, Windass JD, Lansdell SJ, Millar NS (1999) Molecular characterization and imidacloprid selectivity of nicotinic acetylcholine receptor subunits from the peach-potato aphid Myzus persicae. J Neurochem 73:380–389
Huang Y, Williamson MS, Devonshire AL, Windass JD, Lansdell SJ, Millar NS (2000) Cloning, heterologous expression and co-assembly of Mpβ1, a nicotinic acetylcholine receptor subunit from the aphid Myzus persicae. Neurosci Lett 284:116–120
Ihara M, Matsuda K, Otake M, Kuwamura M, Shimomura M, Komai K, Akamatsu M, Raymond V, Sattelle DB (2003) Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology 45:133–144
Ihara M, Matsuda K, Shimomura M, Sattelle DB, Komai K (2004) Super agonist actions of clothianidin and related compounds on the SADβ2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Biosci Biotechnol Biochem 68:761–763
Jones AK, Sattelle DB (2003) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans. Bioessays 26:39–49
Jones AK, Grauso M, Sattelle DB (2005a) The nicotinic acetylcholine receptor gene family of the malaria mosquito, Anopheles gambiae. Genomics 85:176–187
Jones AK, Marshall J, Blake AD, Buckingham SD, Darlison MG, Sattelle DB (2005b) Sgβ1, a novel locust (Schistocerca gregaria) non-α nicotinic acetylcholine receptor-like subunit with homology to the Drosophila melanogaster Dβ1 subunit. Invert Neurosci 5:147–155
Jones AK, Raymond-Delpech V, Thany SH, Gauthier M, Sattelle DB (2006) The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res 16:1422–1430
Kagabu S (1999) Discovery of chloronicotinyl insecticides. In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo, pp 91–106
Kollmeyer WD, Flattum RF, Foster JP, Powell JE, Schroeder ME, Soloway SB (1999) Discovery of the nitromethylene heterocycle insecticides. In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo, pp 71–89
Langley JN (1905) On the reaction of cells and of nerve-endings to certain poisons, chiefly as regarts the reaction of striated muscle to nicotine and curari. J Physiol 33:374–413
Lansdell SJ, Millar NS (2000a) Cloning and heterologous expression of Dα4, a Drosophila neuronal nicotinic acetylcholine receptor subunit: identification of an alternative exon influencing the efficiency of subunit assembly. Neuropharmacology 39:2604–2614
Lansdell SJ, Millar NS (2000b) The influence of nicotinic receptor subunit composition upon agonist, α-bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology 39:671–679
Lansdell SJ, Millar NS (2002) Dβ3, an atypical nicotinic acetylcholine receptor subunit from Drosophila: molecular cloning, heterologous expression and coassembly. J Neurochem 80:1009–1018
Lansdell SJ, Millar NS (2004) Molecular characterisation of Dα6 and Dα7 nicotinic acetylcholine receptor subunits from Drosophila: formation of a high-affinity α-bungarotoxin binding site revealed by expression of subunit chimeras. J Neurochem 90:479–489
Lansdell SJ, Schmitt B, Betz H, Sattelle DB, Millar NS (1997) Temperature-sensitive expression of Drosophila neuronal nicotinic acetylcholine receptors. J Neurochem 68:1812–1819
Le Novère N, Corringer P-J, Changeux J-P (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53:447–456
Lee S-J, Tomizawa M, Casida JE (2003) Neristoxin and cartap neurotoxicity attributable to direct block of the insect nicotinic receptor/channel. J Agric Food Chem 51:2646–2652
Leicht W (1996) Imidacloprid—a chloronicotinyl insecticide: biological activity and agricultural significance. Pfanzenschutz Nachrichten Bayer 49:71–84
Lind RL, Clough MS, Reynolds SE, Earley FGP (1998) [3H]Imidacloprid labels high- and low- affinity nicotinic acetylcholine receptor-like binding sites in the aphid Myzus persicae (Hemiptera: Aphididae). Pestic Biochem Physiol 62:3–14
Lindstrom JM (2003) Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci 998:41–52
Liu M-Y, Casida JE (1993) High affinity binding of [3H]imidacloprid in the insect acetylcholine receptor. Pestic Biochem Physiol 40:40–46
Liu Z, Han Z (2006) Fitness costs of laboratory-selected imidacloprid resistance in brown planthopper, Nilaparvata lugens Stål. Pest Manag Sci 62:279–282
Liu Z, Williamson MS, Lansdell SJ, Denholm I, Han Z, Millar NS (2005) A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc Natl Acad Sci USA 102:8420–8425
Liu Z, Williamson MS, Lansdell SJ, Han Z, Denholm I, Millar NS (2006) A nicotinic acetylcholine receptor mutation (Y151S) causes reduced agonist potency to a range of neonicotinoid insecticides. J Neurochem 99:1273–1281
Loughner RL, Warnock DF, Cloyed RA (2005) Resistance to greenhouse, laboratory, and native populations of western flower thrips to spinosad. HortScience 40:146–149
Maienfisch P, Huerlimann H, Rindlisbacher A, Gsell L, Dettwiler H, Haettenschwiler J, Sieger E, Walti M (2001) The discovery of thiamethoxam: a second generation neonicotinoid. Pest Manag Sci 57:165–176
Mansvelder HD, McGehee DS (2002) Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol 53:606–617
Marshall J, Buckingham SD, Shingai R, Lunt GG, Goosey MW, Darlison MG, Sattelle DB, Barnard EA (1990) Sequence and functional expression of a single α subunit of an insect nicotinic acetylcholine receptor. EMBO J 9:4391–4398
Martin RJ (1997) Modes of action of anthelmintic drugs. Vet J 154:11–34
Matsuda K, Buckingham SD, Freeman JC, Squire MD, Baylis HA, Sattelle DB (1998) Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br J Pharmacol 123:518–524
Matsuda K, Shimomura M, Kondo Y, Ihara M, Hashigami K, Yoshida N, Raymond V, Mongan NP, Freeman JC, Komai K, Sattelle DB (2000) Role of loop D of the α7 nicotinic acetylcholine receptor in its interaction with the insecticide imidacloprid and related neonicotinoids. Br J Pharmacol 130:981–986
Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22:573–580
Millar NS (1999) Heterologous expression of mammalian and insect neuronal nicotinic acetylcholine receptors in cultured cell lines. Biochem Soc Trans 27:944–950
Millar NS (2003) Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans 31:869–874
Millar NS (2004) Nicotinic acetylcholine receptors: pharmacologically diverse drug targets in vertebrates and invertebrates. In: Beadle DJ, Mellor IR, Usherwood PNR (eds) Neurotoxicological targets from functional genomics and proteomics. Society of Chemical Industry, London, pp 25–39
Millar NS (2006) Ligand-gated ion channels. In: Encyclopedia of life sciences. Wiley, Chichester. http://www.els.net/, doi:10.1038/npg.els.0000154
Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD (2006) Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag Sci 62:30–37
Moulton JK, Pepper DA, Dennehy TJ (2000) Beet armyworm (Spodoptera exigua) resistance to spinosad. Pest Manag Sci 56:842–848
Mullin CA, Scott JG (eds) (1992) Molecular mechanisms of insecticide resistance: diversity among insects. American Chemical Society, Washington DC
Nagata K, Iwanaga Y, Shono T, Narahashi T (1997) Modulation of neuronal nicotinic acetylcholine receptor channel by imidacloprid and cartap. Pest Biochem Physiol 59:119–128
Narahashi T (1996) Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol 78:1–14
Nauen R, Bretschneider T (2002) New modes of action of insecticides. Pestic Outlook 13:241–245
Nauen R, Denholm I (2005) Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch Insect Biochem Physiol 58:200–215
Nauen R, Strobel J, Tietjen K, Otsa Y, Erdelen C, Elbert A (1996) Aphicidal activity of imidacloprid against a tobacco feeding strain of Myzus persicae (Homoptera: Aphidade) from Japan closely related to Myzus nicotianae and high resistant to carbamates and organophosphates. Bull Entomol Res 86:165–171
Nauen R, Ebbinghaus-Kintscher U, Elbert A, Jeschke P, Tietjen K (2001) Acetylcholine receptors as sites for developing neonicotinoid insecticides. In: Ishaaya I (ed) Biochemical sites important in insecticide action and resistance. Springer, Berlin, pp 77–105
Nishiwaki H, Nakagawa Y, Kuwamura M, Sato K, Akamatsu M, Matsuda K, Komai K, Miyagawa H (2003) Correlations of the electrophysiological activity of neonicotinoids with their binding and insecticidal activities. Pest Manag Sci 59:1023–1030
Noda M, Takahashi H, Tanabe T, Toyosato M, Furutani Y, Hirose T, Asai M, Inayama S, Miyata T, Numa S (1982) Primary structure of 〈-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 299:793–797
Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Furutana Y, Hirose T, Takashima H, Inayama S, Miyata T, Numa S (1983) Structural homology of Torpedo californica acetylcholine receptor subunits. Nature 302:528–532
Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Hirose T, Asai M, Takashima H, Inayama S, Miyata T, Numa S (1993) Primary structures of ®- and ™-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature 301:251–255
Orr N, Hasler J, Watson G, Mitchell J, Gustafson G, Gifford J, Geng C, Chouinard S, Cook K (2006) Spinosad: from nature to green chemistry to novel mode of action. In: 11th IUPAC International Congress of Pesticide Chemistry Abstracts, Kobe, Japan, p 27
Pap L, Toth A, Karikas S (1997) A survey of the insecticide resistance status of the Colorado potato beetle, Leptinotarsa decemlineata in Hungary between 1987 and 1991. Pestic Sci 49:389–392
Paterson D, Nordberg A (2000) Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61:75–111
Picciotto MR, Zoli M (2002) Nicotinic receptors in aging and dementia. J Neurobiol 53:641–655
Rauch N, Nauen R (2003) Identification of biochemical markers linked to neonicotinoid cross resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). Arch Insect Biochem Physiol 54:165–176
Raymond Delpech V, Ihara M, Coddou C, Matsuda K, Sattelle DB (2003) Action of nereistoxin on recombinant meuronal nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Invert Neurosci 5:29–35
Salgado VL (1998) Studies on the mode of action of spinosad: insect symptoms and physiological correlates. Pest Biochem Physiol 60:91–102
Salgado VL, Saar R (2004) Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J Insect Physiol 50:867–879
Salgado VL, Watson GB, Sheets JJ (1997) Studies on the mode of action of spinosad, the active ingredient in Tracer Insect Control. In: Proceedings of the Beltwide Cotton Production Conference, National Cotton Council, Memphis, TN, USA, pp 1082–1086
Salgado VL, Sheets JJ, Watson GB, Schmidt AL (1998) Studies on the mode of action of spinosad: the internal effective concentration and the concentration dependence of neural excitation. Pest Biochem Physiol 60:103–110
Sattelle DB (1980) Acetylcholine receptors of insects. Adv Insect Physiol 15:215–315
Sattelle DB, Jones AK, Sattelle BM, Matsuda K, Reenan R, Biggin PC (2005) Edit, cut and paste in the nicotinic acetylcholine receptor gene family of Drosophila melanogaster. Bioessays 27:366–376
Sawruk E, Schloss P, Betz H, Schmitt B (1990a) Heterogeneity of Drosophila nicotinic acetylcholine receptors: SAD, a novel developmentally regulated α-subunit. EMBO J 9:2671–2677
Sawruk E, Udri C, Betz H, Schmitt B (1990b) SBD, a novel structural subunit of the Drosophila nicotinic acetylcholine receptor, shares its genomic localization with two α-subunits. FEBS Lett 273:177–181
Schmeltz I (1971) Nicotine and other tobacco alkaloids. In: Jacobson M, Crosby DG (eds) Naturally occurring insecticides. Marcel Dekker, New York, pp 99–136
Schulz R, Sawruk E, Mülhardt C, Bertrand S, Baumann A, Phannavong B, Betz H, Bertrand D, Gundelfinger ED, Schmitt B (1998) Dα3, a new functional a subunit of nicotinic acetylcholine receptors from Drosophila. J Neurochem 71:853–862
Sgard F, Fraser SP, Katkowska MJ, Djamgoz MBA, Dunbar SJ, Windass JD (1998) Cloning and functional characterisation of two novel nicotinic acetylcholine receptor α subunits from the insect pest Myzus persicae. J Neurochem 71:903–912
Shimomura M, Okuda H, Matsuda K, Komai K, Akamatsu M, Sattelle DB (2002) Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br J Pharmacol 137:162–169
Shimomura M, Yokota M, Okumura M, Matsuda K, Akamatsu M, Sattelle DB, Komai K (2003) Combinatorial mutations in loops D and F strongly influence responses of the α7 nicotinic acetylcholine receptor to imidacloprid. Brain Res 991:71–77
Shimomura M, Yokota M, Matsuda K, Sattelle DB, Komai K (2004) Roles of loop C and the loop B–C interval of the nicotinic receptor α subunit in its selective interactions with imidacloprid in insects. Neurosci Lett 363:195–198
Shimomura M, Satoh H, Yokota M, Ihara M, Matsuda K, Sattelle DB (2005) Insect-vertebrate chimeric nicotinic acetylcholine receptors identify a region, loop B to the N-terminus of the Drosophila Dα2 subunit, which contributes to neonicotinoid sensitivity. Neurosci Lett 385:168–172
Soderlund DM, Knipple DC (2003) The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol 33:563–577
Soloway SB (1976) Naturally occurring insecticides. Environ Health Perspect 14:109–117
Soloway SB, Henry AC, Kollmeyer WD, Padgett WM, Powell JE, Roman SA, Tieman CH, Corey RA, Horne CA (1978) Nitromethylene heterocycles as insecticides. In: Pesticide and venom neurotoxicity. Plenum Press, New York, pp 153–158
Sparks TC, Crouse GD, Durst G (2001) Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag Sci 57:896–905
Thany SH, Lenaers G, Crozatier M, Armengaud C, Gauthier M (2003) Identification and localization of the nicotinic acetylcholine receptor alpha3 mRNA in the brain of the honwybee, Apis mellifera. Insect Mol Biol 12:255–262
Thany SH, Crozatier M, Raymond-Delpech V, Gauthier M, Lenaers G (2005) Apisα2, Apisα7-1 and Apisα7-2: three new neuronal nicotinic acetylcholine receptor α-subunits in the honeybee brain. Gene 344:125–132
Thompson GD, Dutton R, Sparks TC (2000) Spinosad—a case study: an example from a natural products discovery programme. Pest Manag Sci 56:696–702
Tomizawa M, Casida JE (2001) Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag Sci 57:914–922
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicity: mechanisms of selective action. Ann Rev Pharmacol Toxicol 45:247–268
Tomizawa M, Lee DL, Casida JE (2000) Neonicotinoid insecticides: molecular features conferring selectivity for insect versus mammalian nicotinic receptors. J Agric Food Chem 48:6016–6024
Tomizawa M, Zhang N, Durkin KA, Olmstead MM, Casida JE (2003) The neonicotinoid electronegative pharmacophore plays the crucial role in the high affinity and selectivity for the Drosophila nicotinic receptor: an anomaly for the nicotinoid cation-π interaction model. Biochem 42:7819–7827
Tomizawa M, Millar NS, Casida JE (2005) Pharmacological profiles of recombinant and native insect nicotinic acetylcholine receptors. Insect Biochem Mol Biol 35:1347–1355
Ujváry I (1999) Nicotine and other insecticidal alkaloids. In: Yamamoto I, Casida JE (eds) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo, pp 29–69
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol 346:967–989
Usherwood PNR (1994) Insect glutamate receptors. Adv Insect Physiol 24:309–341
Vais H, Williamson MS, Devonshire AL, Usherwood PN (2001) The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Manag Sci 57:877–888
Wakita T, Kinoshita K, Yamada E, Yasui N, Kawahara N, Naoi A, Nakaya M, Ebihara K, Matsuno H, Kodaka K (2003) The discovery of dinotefuran: a novel neonicotinoid. Pest Manag Sci 59:1016–1022
Watson GB (2001) Actions of insecticidal spinosyns on γ-aminobutyric acid responses from small-diameter cockroach neurons. Pest Biochem Physiol 71:20–28
Weiland S, Bertrand D, Leonard S (2000) Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behav Brain Res 113:43–56
Wiesner P, Kayser H (2000) Characterization of nicotinic acetylcholine receptors from the insects Aphis craccivora, Myzus persicae, and Locusta migratoria by radioligand binding assays: relation to thiamethoxam action. J Biochem Mol Toxicol 14:221–230
Wyss CF, Young HP, Shukla J, Roe RM (2003) Biology and genetics of a laboratory strain of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae), highly resistant to spinosad. Crop Prot 22:307–314
Yamamoto I, Casida JE (1999) Nicotinoid insecticides and the nicotinic acetylcholine receptor. Springer, Tokyo
Young HP, Bailey WD, Roe RM (2003) Spinosad selection of a laboratory strain of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae), and characterization of resistance. Crop Prot 22:265–273
Zewen L, Zhaojun H, Yinchang W, Lingchun Z, Hongwei Z, Chengjun L (2003) Selection for imidacloprid resistance in Nilaparvata lugens: cross-resistance patterns and possible mechanisms. Pest Manag Sci 59:1355–1359
Zhang A, Kayser H, Maienfisch P, Casida JE (2000) Insect nicotinic acetylcholine receptor: conserved neonicotinoid specificity of [3H]imidacloprid binding site. J Neurochem 75:1294–1303
Zhao J-Z, Bishop BA, Grafius EJ (2000) Inheritance and synergism of resistance to imidacloprid in the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 93:1508–1514
Zhao J-Z, Collins HL, Li YX, Mau RF, Thompson GD, Hertlein M, Andaloro JT, Boykin R, Shelton AM (2006) Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. J Econ Entomol 99:176–181
Zwart R, Oortgiesen M, Vijverberg HPM (1992) The nitromethylene heterocycle 1-(pyridin-3-yl-methyl)-2-nitomethylene-imidazolidine distinguishes mammalian from insect nicotinic receptor subtypes. Eur J Pharmacol 228:165–169
Zwart R, Oortgiesen M, Vijverberg HPM (1994) The nitromethylene heterocycles: selective agonists of nicotinic receptors in locust neurons compared to mouse NE-115 and BC3H1 cells. Pestic Biochem Physiol 48:202–213
Acknowledgements
We thank numerous colleagues, especially Stuart Lansdell, Zewen Liu, Ralf Nauen and Martin Williamson, whose work and discussions we have drawn upon in the preparation of this review. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom. NSM acknowledges funding from the BBSRC, the Royal Society and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Millar, N.S., Denholm, I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert Neurosci 7, 53–66 (2007). https://doi.org/10.1007/s10158-006-0040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-006-0040-0