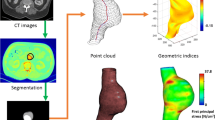
Abstract
Both the clinically established diameter criterion and novel approaches of computational finite element (FE) analyses for rupture risk stratification of abdominal aortic aneurysms (AAA) are based on assumptions of population-averaged, uniform material properties for the AAA wall. The presence of inter-patient and intra-patient variations in material properties is known, but has so far not been addressed sufficiently. In order to enable the preoperative estimation of patient-specific AAA wall properties in the future, we investigated the relationship between non-invasively assessable clinical parameters and experimentally measured AAA wall properties. We harvested n = 163 AAA wall specimens (n = 50 patients) during open surgery and recorded the exact excision sites. Specimens were tested for their thickness, elastic properties, and failure loads using uniaxial tensile tests. In addition, 43 non-invasively assessable patient-specific or specimen-specific parameters were obtained from recordings made during surgery and patient charts. Experimental results were correlated with the non-invasively assessable parameters and simple regression models were created to mathematically describe the relationships. Wall thickness was most significantly correlated with the metabolic activity at the excision site assessed by PET/CT (ρ = 0.499, P = 4 × 10−7) and to thrombocyte counts from laboratory blood analyses (ρ = 0.445, P = 3 × 10−9). Wall thickness was increased in patients suffering from diabetes mellitus, while it was significantly thinner in patients suffering from chronic kidney disease (CKD). Elastic AAA wall properties had significant correlations with the metabolic activity at the excision site (PET/CT), with existent calcifications, and with the diameter of the non-dilated aorta proximal to the AAA. Failure properties (wall strength and failure tension) had correlations with the patient’s medical history and with results from laboratory blood analyses. Interestingly, AAA wall failure tension was significantly reduced for patients with CKD and elevated blood levels of potassium and urea, respectively, both of which are associated with kidney disease. This study is a first step to a future preoperative estimation of AAA wall properties. Results can be conveyed to both the diameter criterion and FE analyses to refine rupture risk prediction. The fact that AAA wall from patients suffering from CKD featured reduced failure tension implies an increased AAA rupture risk for this patient group at comparably smaller AAA diameters.
Similar content being viewed by others
References
Alberti KGMM, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetes Med 15(7): 539–553
Breeuwer M, de Putter S, Kose U, Speelman L, Visser K, Gerritsen F, Hoogeveen R, Krams R, van den Bosch H, Buth J, Gunther T, Wolters B, van Dam E, van de Vosse F (2008) Towards patient-specific risk assessment of abdominal aortic aneurysm. Med Biol Eng Comp 46(11): 1085–1095
Choke E, Cockerill G, Wilson WRW, Sayed S, Dawson J, Loftus I, Thompson MM (2005) A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 30(3): 227–244
CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney disease: improving global outcomes (kdigo) CKD-MBD work group. Kidney Int Suppl 113:1–130
Defawe OD, Hustinx R, Defraigne JO, Limet R, Sakalihasan N (2005) Distribution of F-18 fluorodeoxyglucose (F-18 FDG) in abdominal aortic aneurysm: high accumulation in macrophages seen on PET imaging and immunohistology. Clin Nucl Med 30(5): 340–341
Doyle BJ, Hoskins PR, McGloughlin TM (2011) Commentary: computational rupture prediction of aaas: what needs to be done next?. J Endovasc Ther 18(2): 226–229
Fillinger MF, Marra SP, Raghavan ML, Kennedy FE (2003) Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 37(4): 724–732
Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P (2011) Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol 58(6): 603–614
Forsdahl SH, Singh K, Solberg S, Jacobsen BK (2009) Risk factors for abdominal aortic aneurysms. Circulation 119(16): 2202–2208
Fung YC (1993) Biomechanics—mechanical properties of living tissues, 2nd edn. Springer, New York
Gasser TC, Görgülü G, Folkesson M, Swedenborg J (2008) Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J Vasc Surg 48(1): 179–188
Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J (2010) Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc Surg 40(2): 176–185
Gee MW, Reeps C, Eckstein HH, Wall WA (2009) Prestressing in finite deformation abdominal aortic aneurysm simulation. J Biomech 42(11): 1732–1739
Gee MW, Förster C, Wall WA (2010) A computational strategy for prestressing patient specific biomechanical problems under finite deformation. Int J Numer Meth Biomed Eng 26(1): 52–72
Greenhalgh RM (2004) Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 364(9437): 843–848
Holzapfel GA (2000) Nonlinear solid mechanics: a contiuum approach for engineering. Wiley, Chichester
Holzapfel GA, Ogden RW (2010) Constitutive modelling of arteries. Proc R Soc A 466: 1551–1597
Humphrey JD (2002) Cardiovascular solid mechanics—cells, tissues, and organs. Springer, New York
Humphrey JD, Holzapfel GA (2012) Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 45(5): 805–814
Humphrey JD, Rajagopal KR (2003) A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech Model Mechanobiol 2(2): 109–126
Humphrey JD, Taylor CA (2008) Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng 10(1): 221–246
Hyhlik-Dürr A, Krieger T, Geisbüsch P, Kotelis D, Able T, Böckler D (2011) Reproducibility of deriving parameters of AAA rupture risk from patient-specific 3D finite element models. J Endovasc Ther 18(3): 289–298
Kotze C, Groves A, Menezes L, Harvey R, Endozo R, Kayani I, Ell P, Yusuf S (2011) What is the relationship between 18F-FDG aortic aneurysm uptake on PET/CT and future growth rate?. Eur J Nucl Med Mol Imaging 38(8): 1493–1499
Li ZY, U-King-Im J, Tang TY, Soh E, See TC, Gillard JH (2008) Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg 47(5): 928–935
Lim ST, Kim YK, Hwang JK, Kim SD, Park SC, Won YS, Park JS, Kim JI, Yun SS, Moon IS (2011) A clinical consideration of abdominal aortic aneurysm rupture. Korean J Vasc Endovasc Surg 27(3): 103–107
Maier A, Gee MW, Reeps C, Eckstein HH, Wall WA (2010) Impact of calcifications on patient-specific wall stress analysis of abdominal aortic aneurysms. Biomech Model Mechanobiol 9(5): 511–521
Maier A, Gee MW, Reeps C, Pongratz J, Eckstein HH, Wall WA (2010) A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann Biomed Eng 38(10): 3124–3134
Maier A, Essler M, Gee MW, Eckstein HH, Wall WA, Reeps C (2012) Correlation of biomechanics to tissue reaction in aortic aneurysms assessed by finite elements and [18F]-fluorodeoxyglucose-PET/CT. Int J Numer Meth Biomed Eng 28(4): 456–471
Marini G, Maier A, Reeps C, Eckstein HH, Wall WA, Gee MW (2012) A continuum description of the damage process in the arterial wall of abdominal aortic aneurysms. Int J Numer Meth Biomed Eng 28(1): 87–99
Ockert S, Boeckler D, Allenberg J, Schumacher H (2007) Rupturiertes abdominelles Aortenaneurysma. Gefaesschirurgie 12(5): 379–391
Okamoto R, Wagenseil J, DeLong W, Peterson S, Kouchoukos N, Sundt T (2002) Mechanical properties of dilated human ascending aorta. Ann Biomed Eng 30(5): 624–635
Pelisek J, Assadian A, Sarkar O, Eckstein HH, Frank H (2010) Carotid plaque composition in chronic kidney disease: a retrospective analysis of patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg 39(1): 11–16
Pelisek J, Hahntow IN, Eckstein HH, Ockert S, Reeps C, Heider P, Luppa PB, Frank H (2011) Impact of chronic kidney disease on carotid plaque vulnerability. J Vasc Surg 54(6): 1643–1649
Polzer S, Gasser T, Swedenborg J, Bursa J (2011) The impact of intraluminal thrombus failure on the mechanical stress in the wall of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 41(4): 467–473
Raghavan ML, Vorp DA (2000) Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech 33(4): 475–482
Raghavan ML, Webster MW, Vorp DA (1996) Ex vivo biomechanical behavior of abdominal aortic aneurysm: assessment using a new mathematical model. Ann Biomed Eng 24(5): 573–582
Raghavan ML, Hanaoka MM, Kratzberg JA, Higuchi MdL, da Silva ES (2011a) Biomechanical failure properties and microstructural content of ruptured and unruptured abdominal aortic aneurysms. J Biomech 44(13): 2501–2507
Raghavan ML, Lin K, Ramachandran M, Nadereishvili A, Amelon R, Lu J (2011b) Planar radial extension for constitutive modeling of anisotropic biological soft tissues. Int J Struct Changes Sol 3(2): 23–31
Rausch SMK, Martin C, Bornemann PB, Uhlig S, Wall WA (2011) Material model of lung parenchyma based on living precision-cut lung slice testing. J Mech Behav Biomed Mater 4(4): 583–592
Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ (2008) Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg 48(2): 417–423
Reeps C, Gee MW, Maier A, Pelisek J, Gurdan M, Wall WA, Mariss J, Eckstein HH, Essler M (2009) Glucose metabolism in the vessel wall correlates with mechanical instability and inflammatory changes in a patient with a growing aneurysm of the abdominal aorta. Circ Cardiovasc Imaging 2(6): 507–509
Reeps C, Gee M, Maier A, Gurdan M, Eckstein HH, Wall WA (2010) The impact of model assumptions on results of computational mechanics in abdominal aortic aneurysm. J Vasc Surg 51(3): 679–688
Royston P, Ambler G, Sauerbrei W (1999) The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28(5): 964–974
Sakalihasan N, Hustinx R, Limet R (2004) Contribution of PET scanning to the evaluation of abdominal aortic aneurysm. Sem Vasc Surg 17(2): 144–153
Sauerbrei W, Meier-Hirmer C, Benner A, Royston P (2006) Multivariable regression model building by using fractional polynomials: description of SAS, STATA and R programs. Comput Stat Data Anal 50(12): 3464–3485
The UK Small Aneurysm Trial Participants with Brown LC and Powell JT (1999) Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Ann Surg 230(3):289–297 (2006)
Thubrikar MJ, Labrosse F, Robicsek J, Al-Soudi B, Fowler M (2001) Mechanical properties of abdominal aortic aneurysm wall. J Med Eng Technol 25(4): 133–142
Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX (2006) Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17(7): 2034–2047
Truijers M, Kurvers HAJM, Bredie SJH, Oyen WJG, Blankensteijn JD (2008) In vivo imaging of abdominal aortic aneurysms: increased FDG uptake suggests inflammation in the aneurysm wall. J Endovasc Ther 15(4): 462–467
Vallabhaneni S, Gilling-Smith G, How T, Carter S, Brennan J, Harris P (2004) Heterogeneity of tensile strength and matrix metalloproteinase activity in the wall of abdominal aortic aneurysms. J Endovasc Ther 11: 494–502
Vande Geest JP, Sacks MS, Vorp DA (2006) The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J Biomech 39(7): 1324–1334
Vande Geest JP, Wang DHJ, Wisniewski S, Makaroun M, Vorp D (2006) Towards a noninvasive method for determination of patient-specific wall strength distribution in abdominal aortic aneurysms. Ann Biomed Eng 34(7): 1098–1106
Wall WA, Gee MW (2010) Baci: a parallel multiphysics simulation environment. Technical report, Institute for Computational Mechanics, Technische Universität München
Watton P, Hill N (2009) Evolving mechanical properties of a model of abdominal aortic aneurysm. Biomech Model Mechanobiol 8(1): 25–42
Xenos M, Rambhia S, Alemu Y, Einav S, Labropoulos N, Tassiopoulos A, Ricotta J, Bluestein D (2010) Patient-based abdominal aortic aneurysm rupture risk prediction with fluid structure interaction modeling. Ann Biomed Eng 38(11): 3323–3337
Zeinali-Davarani S, Raguin L, Vorp DA, Baek S (2011) Identification of in vivo material and geometric parameters of a human aorta: toward patient-specific modeling of abdominal aortic aneurysm. Biomech Model Mechanobiol 10(5): 689–699
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reeps, C., Maier, A., Pelisek, J. et al. Measuring and modeling patient-specific distributions of material properties in abdominal aortic aneurysm wall. Biomech Model Mechanobiol 12, 717–733 (2013). https://doi.org/10.1007/s10237-012-0436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0436-1