Abstract
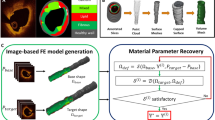
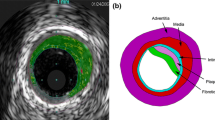
Computational models have been used to calculate plaque stress and strain for plaque progression and rupture investigations. An intravascular ultrasound (IVUS)-based modeling approach is proposed to quantify in vivo vessel material properties for more accurate stress/strain calculations. In vivo Cine IVUS and VH-IVUS coronary plaque data were acquired from one patient with informed consent obtained. Cine IVUS data and 3D thin-slice models with axial stretch were used to determine patient-specific vessel material properties. Twenty full 3D fluid–structure interaction models with ex vivo and in vivo material properties and various axial and circumferential shrink combinations were constructed to investigate the material stiffness impact on stress/strain calculations. The approximate circumferential Young’s modulus over stretch ratio interval [1.0, 1.1] for an ex vivo human plaque sample and two slices (S6 and S18) from our IVUS data were 1631, 641, and 346 kPa, respectively. Average lumen stress/strain values from models using ex vivo, S6 and S18 materials with 5 % axial shrink and proper circumferential shrink were 72.76, 81.37, 101.84 kPa and 0.0668, 0.1046, and 0.1489, respectively. The average cap strain values from S18 material models were 150–180 % higher than those from the ex vivo material models. The corresponding percentages for the average cap stress values were 50–75 %. Dropping axial and circumferential shrink consideration led to stress and strain over-estimations. In vivo vessel material properties may be considerably softer than those from ex vivo data. Material stiffness variations may cause 50–75 % stress and 150–180 % strain variations.








Similar content being viewed by others
References
Barrett SRH, Sutcliffe MPF, Howarth S, Li ZY, Gillard JH (2009) Experimental measurement of the mechanical properties of carotid atherothrombotic plaque fibrous cap. J Biomech 42(11):1650–1655
Bluestein D, Alemu Y, Avrahami I, Gharib M, Dumont K, Ricotta JJ, Einav S (2008) Influence of microcalcifications on vulnerable plaque mechanics using FSI modeling. J Biomech 41(5):1111–1118
Cardoso L, Weinbaum S (2014) Changing views of the biomechanics of vulnerable plaque rupture: a review. Ann Biomed Eng 42(2):415–431
Chai CK, Akyildiz AC, Speelman L, Gijsen FJ, Oomens CW, van Sambeek MR, van der Lugt A, Baaijens FP (2015) Local anisotropic mechanical properties of human carotid atherosclerotic plaques—characterisation by micro-indentation and inverse finite element analysis. J Mech Behav Biomed Mater 43:59–68
Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD et al (2012) Detection of high-risk atherosclerotic plaque: report of the NHLBI working group on current status and future directions. JACC Cardiovasc Imaging 5(9):941–955
Friedman MH, Krams R, Chandran KB (2010) Flow interactions with cells and tissues: cardiovascular flows and fluid–structure interactions. Ann Biomed Eng 38(3):1178–87
Fuster V (1998) The vulnerable atherosclerotic plaque: understanding, identification, and modification. In: Fuster V, Cornhill JF, Dinsmore RE, Fallon JT, Insull W, Libby P et al (eds) AHA monograph series. Futura Publishing, Armonk
Gee MW, Reeps C, Eckstein HH, Wall WA (2009) Prestressing in finite deformation abdominal aortic aneurysm simulation. J Biomech 42(11):1732–1739
Holzapfel GA (2000) Nonlinear solid mechanics: a continuum approach for engineering. Wiley, Chichester
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast Phys Sci Sol 61(1–3):1–48
Holzapfel GA, Sommer G, Regitnig P (2004) Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J Biomech Eng 126(5):657–665
Holzapfel GA, Stadler M, Schulze-Bause CAJ (2002) A layer-specific three-dimensional model for the simulation of balloon angioplasty using magnetic resonance imaging and mechanical testing. Ann Biomed Eng 30(6):753–767
Huang X, Yang C, Yuan C, Liu F, Canton G, Zheng J, Woodard PK, Sicard GA, Tang D (2009) Patient-specific artery shrinkage and 3D zero-stress state in multi-component 3D FSI models for carotid atherosclerotic plaques based on in vivo MRI data. Mol Cell Biomech 6(2):121–134
Humphrey JD (2002) Cardiovascular solid mechanics. Springer, New York
Karimi A, Navidbakhsh M, Shojaeic A (2015) A combination of histological analyses and uniaxial tensile tests to determine the material coefficients of the healthy and atherosclerotic human coronary arteries. Tissue Cell 47:152–158
Kural MH, Cai MC, Tang D, Gwyther T, Zheng J, Billiar KL (2012) Planar biaxial characterization of diseased human coronary and carotid arteries for computational modeling. J Biomech 45(5):790–798 (NIHNSID: 344897)
Liu H, Canton G, Yuan C, Yang C, Billiar KL, Teng Z, Hoffman AH, Tang D (2012) Using in vivo Cine and 3D multi-contrast MRI to determine human atherosclerotic carotid artery material properties and circumferential shrinkage rate and their impact on stress/strain predictions. J Biomech Eng 34(1):011008. doi:10.1115/1.4005685
Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG (2001) American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS): a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 37(5):1478–1492. doi:10.1016/S0735-1097(01)01175-5
Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG (2002) Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106(17):2200–2206. doi:10.1161/01.CIR.0000035654.18341
Nieuwstadt HA, Fekkes S, Hansen HH, de Korte CL, van der Lugt A, Wentzel JJ, van der Steen AF, Gijsen FJ (2015) Carotid plaque elasticity estimation using ultrasound elastography, MRI, and inverse FEA—a numerical feasibility study. Med Eng Phys 37(8):801–807. doi:10.1016/j.medengphy.2015.06.003 (Epub 2015 Jun 27)
Ohayon J, Dubreuil O, Tracqui P, Floc’h SL, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G (2007) Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 293:H1987–H1996
Pandit A, Lu X, Wang C, Kassab GS (2005) Biaxial elastic material properties of porcine coronary media and adventitia. Am J Physiol Heart Circ Physiol 288(6):H2581–H2587
Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP (2011) Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124(7):779–788
Speelman L, Akyildiz AC, Den Adel B, Wentzel JJ, Van der Steen AF, Virmani R, Van der Weerd L, Jukema JW, Poelmann RE, Van Brummelen EH, Gijsen FJ (2011) Initial stress in biomechanical models of atherosclerotic plaques. J Biomech 44(13):2376–2382
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and the histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, AHA. Circulation 92:1355–1374
Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Nakamura S, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimach F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL (2012) PREDICTION Investigators. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation 126(2):172–181
Tang D, Kamm RD, Yang C, Zheng J, Canton G, Bach RG et al (2014) Image-based modeling for better understanding and assessment of atherosclerotic plaque progression and vulnerability: data, modeling, validation, uncertainty and predictions. J Biomech 47(4):834–846
Tang D, Teng Z, Canton G, Yang C, Ferguson M, Huang X, Zheng J, Woodard PK, Yuan C (2009) Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses. An in vivo MRI-based 3D fluid–structure interaction study. Stroke. 40:3258–3263 (Featured article on MDlinx.com)
Tang D, Yang C, Mondal S, Liu F, Canton G, Hatsukami TS, Yuan C (2008) A negative correlation between human carotid atherosclerotic plaque progression and plaque wall stress: in vivo MRI-based 2D/3D FSI models. J Biomech 41(4):727–736 (Featured article by the Society for Heart Attack Prevention and Eradication (SHAPE), 2008)
Teng Z, Tang D, Zheng J, Woodard PK, Hoffman AH (2009) An experimental study on the ultimate strength of the adventitia and media of human atherosclerotic carotid arteries in circumferential and axial directions. J Biomech 42(15):2535–2539
Teng Z, Zhang Y, Huang Y, Feng J, Yuan J, Lu Q, Sutcliffe MP, Brown AJ, Jing Z, Gillard JH (2014) Material properties of components in human carotid atherosclerotic plaques: a uniaxial extension study. Acta Biomater S1742–7061(14):00379-1
Walsh MT, Cunnane EM, Mulvihill JJ, Akyildiz AC, Gijsen FJ, Holzapfel GA (2014) Uniaxial tensile testing approaches for characterization of atherosclerotic plaques. J Biomech 47(4):793–804. doi:10.1016/j.jbiomech.2014.01.017 (Epub 2014 Jan 14)
Wang L, Zheng J, Maehara A, Yang C, Billiar KL, Wu Z, Bach R, Muccigrosso D, Mintz GS, Tang D (2015) Morphological and stress vulnerability indices for human coronary plaques and their correlations with cap thickness and lipid percent: an IVUS-based fluid–structure interaction multi-patient study. PLoS Comput Biol 11(12):e1004652. doi:10.1371/journal.pcbi.1004652
Yang C, Bach R, Zheng J, El Naqa I, Woodard PK, Teng Z, Billiar KL, Tang D (2009) In vivo IVUS-based 3D fluid structure interaction models with cyclic bending and anisotropic vessel properties for human atherosclerotic coronary plaque mechanical analysis. IEEE Trans Biomed Eng 56(10):2420–2428
Yang C, Tang D, Yuan C, Hatsukami TS, Zheng J, Woodard PK (2007) In vivo/ ex vivo MRI-based 3D models with fluid–structure interactions for human atherosclerotic plaques compared with fluid/wall-only models. CMES Comput Model Eng Sci 19(3):233–245
Acknowledgments
This research was supported in part by NIH Grant R01 EB004759 and a Jiangsu Province Science and Technology Agency grant BE2016785. Consultations and guidance from Professor Roger Kamm at MIT are happily acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to disclose.
Additional information
Xiaoya Guo and Jian Zhu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, X., Zhu, J., Maehara, A. et al. Quantify patient-specific coronary material property and its impact on stress/strain calculations using in vivo IVUS data and 3D FSI models: a pilot study. Biomech Model Mechanobiol 16, 333–344 (2017). https://doi.org/10.1007/s10237-016-0820-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0820-3