Abstract
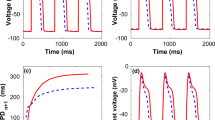
Experimental studies have shown that cardiac fibroblasts are electrically inexcitable, but can contribute to electrophysiology of myocardium in various manners. The aim of this computational study was to give insights in the electrophysiological role of fibroblasts and their interaction with myocytes. We developed a mathematical model of fibroblasts based on data from whole-cell patch clamp and polymerase chain reaction (PCR) studies. The fibroblast model was applied together with models of ventricular myocytes to assess effects of heterogeneous intercellular electrical coupling. We investigated the modulation of action potentials of a single myocyte varying the number of coupled fibroblasts and intercellular resistance. Coupling to fibroblasts had only a minor impact on the myocyte’s resting and peak transmembrane voltage, but led to significant changes of action potential duration and upstroke velocity. We examined the impact of fibroblasts on conduction in one-dimensional strands of myocytes. Coupled fibroblasts reduced conduction and upstroke velocity. We studied electrical bridging between ventricular myocytes via fibroblast insets for various coupling resistors. The simulations showed significant conduction delays up to 20.3 ms. In summary, the simulations support strongly the hypothesis that coupling of fibroblasts to myocytes modulates electrophysiology of cardiac cells and tissues.

















Similar content being viewed by others
References
Antzelevitch, C., and A. Lukas. Reflection and circus movement reentry in isolated atrial and ventricular tissue. In: Electrophysiology and Pharmacology of the Heart, edited by K. H. Dangman and D. S. Miura. Marcel Dekker, 1991, Chapter 11, pp. 251–275.
Brown R. D., Ambler S. K., Mitchell M. D., Long C. S. (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu. Rev. Pharmacol. Toxicol. 45:657–687
Cabo C., Barr R. C. (1992) Reflection after delayed excitation in a computer model of a single fiber. Circ. Res. 71:260–270
Camelliti P., Devlin G. P., Matthews K. G., Kohl P., Green C. R. (2004) Spatially and temporally distinct expression of connexins after sheep ventricular infarction. Cardiovas. Res. 62:415–425
Camelliti P., Green C. R., LeGrice I., Kohl P. (2004) Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ. Res. 94(6):828–835
Camelliti, P., and P. Kohl. Myocyte/fibroblast 2D structured cardiac tissue model. J. Phyiol. 552P:P36, 2003.
Camelliti P., McCulloch A. D., Kohl P. (2005) Microstructured cocultures of cardiac myocytes and fibroblasts: a two-dimensional in vitro model of cardiac tissue. Microsc. Microanal. 11:249–259
Chilton L., Ohya S., Freed D., George E., Drobic V., Shibukawa Y., MacCannel K. A., Imaizumi Y., Clark R. B., Dixon M. C., Giles W. R. (2005) K+ currents regulate the resting membrane potential, proliferation, and contractile response in ventricular fibroblasts and myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 288:H2931–H2939
Cranefield P. F., Hoffmann B. F. (1971) Conduction of the cardiac impulse: II. summation and inhibition. Circ. Res. 28:220–233
Cranefield P. F., Klein H. O., Hoffmann B. F. (1971) Conduction of the cardiac impulse: I. Delay, block, and one-way block in depressed Purkinje fibers. Circulation 28:199–219
Cranefield P. F., Wit A. L., Hoffmann B. F. (1971) Conduction of the cardiac impulse: III. Characteristics of slow conduction. Circ. Res. 59:227–246
Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. (1988) Collagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell. Cardiol. 20:267–276
Fleischhauer J., Lehmann L., Kĺeber A. G. (1995) Electrical resistances of interstitial and microvascular space a determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation 92:587–594
Gaudesius G., Miragoli M., Thomas S. P., Rohr S. (2003) Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 93:421–428
Geneser, S. E., R. M. Kirby, and F. B. Sachse. Sensitivity analysis of cardiac electrophysiological models using polynomial chaos. Proc. IEEE EMBS 4:4042–4045, 2005.
Goldsmith E. C., Hoffman A., Morales M. O., Potts J. D., Price R. L., McFadden A., Rice M., Borg T. K. (2004) Organization of fibroblasts in the heart. Dev. Dynam. 230:787–794
Goshima K. (1969) Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in a monolayer culture. Exp. Cell Res. 58:420–426
Harks E. G., Torres J. J., Cornelisse L. N., Ypey D. L., Theuvenet A. P. (2003) Ionic basis for excitability of normal rat kidney (NRK) fibroblasts. J. Cell Physiol. 196(3):493–503
B. Hille. Ionic Channels of Excitable Membranes, 2nd edition. Sinauer Associates, 1992.
Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. (1969) Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog. Brain Res. 31:283–311
Iyer V., Mazhari R., Winslow R. L. (2004) A computational model of the human left-ventricular epicardial myocyte. Biophys. J. 87(3):1507–1525
Kamkin A., Kiseleva I., Lozinsky I., Scholz H. (2005) Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res. Cardiol. 100:337–345
Kamkin A., Kiseleva I., Wagner K.-D., Pylaev A., Leiterer K. P., Theres H., Scholz H., Günther J., Isenberg G. (2002) A possible role for atrial fibroblasts in postinfarction bradycardia. Am. J. Physiol. 282:H842–H849
Kiseleva I., Kamkin A., Pylaev A., Kondratjev D., Leiterer K. P., Theres H., Wagner K. D., Persson P. B., Gunther J. (1998) Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J. Mol. Cell. Cardiol. 30(6):1083–1093
Klemic K. G., Durand D. M., Jones S. W. (1998) Activation kinetics of the delayed rectifier potassium current of bullfrog sympathetic neurons. J. Neurophysiol. 79:2345–2357
Kohl P., Camelliti P., Burton F. L., Smith G. L. (2005) Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J. Electrocardiol. 38:45–50
Kohl P., Kamkin A. G., Kiseleva I. S., Noble D. (1994) Mechanosensitive fibroblasts in the sino-atrial node region of the rat heart: interaction with cardiomyocytes and possible role. Exp. Physiol. 79:943–956
Kohl P., Noble D. (1996) Mechanosensitive connective tissue: Potential influence on heart rhythm. Cardiovasc. Res. 32:62–68
Luo C.-H., Rudy Y. (1994) A dynamic model of the ventricular cardiac action potential: I. Simulations of ionic currents and concentration changes. Circ. Res. 74(6):1071–1096
Luo C.-H., Rudy Y. (1994) A Dynamic Model of the Ventricular Cardiac Action Potential: II. Afterdepolarizations, Triggered Activity, and Potentiation. Circ. Res. 74(6):1097–1113
Manabe I., Shindo T., Nagai R. (2005) Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ. Res. 91:1103–1113
Nishimura S., Kawai Y., Nakajima T., Hosoya Y., Fujita H., Katoh M., Yamashita H., Nagai R., Sugiura S. (2006) Membrane potential of rat ventricular myocytes responds to axial stretch in phase, amplitude and speed-dependent manners. Cardiovasc. Res. 72:403–411
Noble D., Varghese A., Kohl P., Noble P. (1998) Improved guinea-pig ventricular cell model incorporating a diadic space, I Kr and I Ks, and length- and tension-dependent processes. Can. J. Cardiol. 14(1):123–134
Pandit S. V., Clark R. B., Giles W. R., Demir S. S. (2001) A mathematical model of action potential heterogeneity in adult left ventricular myocytes. Biophys. J. 81:3029–3051
Penefsky Z. J., Hoffman B. F. (1963) Effects of stretch on mechanical and electrical properties of cardiac muscle. Am. J. Physiol. 204(3):433–438
Press W. H., Teukolsky S. A., Vetterling W. T., Flannery B. P. (1992) Numerical Recipes in C, 2nd edition. Cambridge University Press, Cambridge, New York, Melbourne
Rook M. B., van Ginneken A. C. G., de Jonge B., El Aoumari A., Gros D., Jongsma H. J. (1992) Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am. J. Physiol. 263:C959–C977
Rudy Y. (2004) Conductive bridges in cardiac tissue: a beneficial role or an arrhythmogenic substrate. Circ. Res. 94:709–711
Ruknudin A., Sachs F., Bustamante J. O. (1993) Stretch-activated ion channels in tissue-cultured chick heart. Am. J. Physiol. Heart Circ. Physiol. 264(33):H960–972
Sachse, F. B. Computational Cardiology: Modeling of Anatomy, Electrophysiology, and Mechanics. LNCS 2966. Heidelberg:Springer Press, 2004.
Sano T., Takayama N., Shimamoto T. (1959) Directional difference of conduction velocity in the cardiac ventricular syncytium studied by microelectrodes. Circ. Res. 7:262–267
Shaw R. M., Rudy Y. (1997) Ionic mechanisms of propagation in cardiac tissue: roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ. Res. 81:727–741
Shibukawa Y., Chilton L., MacCannell K. A., Clark R. B., Giles W. R. (2005) K+ currents activated by depolarization in cardiac fibroblasts. Biophys. J. 88:3924–3935
Spear J. F., Moore E. N. (1972) Stretch-Induced Excitation and Conduction Disturbances in the Isolated Rat Myocardium. J. Electrocardiol. 5(1):15–24
Torres J. J., Cornelisse L. N., Harks E. G. A., van Meerwijk W. P. M., Theuvenet A. P. R., Ypey D. L. (2004) Modeling action potential generation and propagation in NRK fibroblasts. Am. J. Physiol. 287:H851–H865
Vasquez C., Moreno A. P., Berbari E. J. (2004) Modeling fibroblast–mediated conduction in the ventricle. Proc. CiC 31:349–352
Wit A. L., Cranefield P. F., Hoffman B. F. (1972) Slow Conduction and Reentry in the Ventricular Conduction System: II. Single and sustained circus movement in networks of canine and bovine Purkinje fibers. Circ. Res. 30:11–22
Wit A. L., Hoffman B. F., Cranefield P. F. (1972) Slow Conduction and Reentry in the Ventricular Conduction System: I. Return extrasystole in the canine Purkinje fibers. Circ. Res. 30(1):1–10
Zagotta W. N., Aldrich R. W. (1990) Voltage-dependent gating of Shaker A-type potassium channels in drosophila muscle. J. Gen. Physiol. 95(1):29–60
Acknowledgments
This work has been supported by the Richard A. and Nora Eccles Fund for Cardiovascular Research, awards from the Nora Eccles Treadwell Foundation (FBS, APM, JAA), and NIH/NHLBI, HL63969 (APM). We thank Ms. Catherine Lloyd, University of Auckland, and Dr. Alan Garny, University of Oxford, for implementing a CellML version of the fibroblast model. We acknowledge the support of Prof. James B. Bassingthwaighte and Dr. Brian Carlson, University of Washington, for implementation of the fibroblast and myocyte-fibroblast models in JSim and including these in the model repository of the NSR Physiome Project (http://www.physiome.org/model/doku.php?id=Cell_Physiology:Fibroblast:model_index and http://www.physiome.org/model/doku.php?id=Cell_Physiology:Action_potential:Myocyte_fibroblast_coupling:model_index).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sachse, F.B., Moreno, A.P. & Abildskov, J. . Electrophysiological Modeling of Fibroblasts and their Interaction with Myocytes. Ann Biomed Eng 36, 41–56 (2008). https://doi.org/10.1007/s10439-007-9405-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9405-8