Abstract
Non-physiological mechanobiological stimuli typically occur in tumors and are considered to promote cancer spreading. Non-fluid related pressure (solid stress), which arises as tumors grow against adjacent tissues, is among the least studied endogenous stimuli due to challenges in replicating the in vivo environment. To this end, the novel devices well-pressor and the videomicroscopy-compatible optic-pressor were developed to exert precise compressive strain on cells in 3D gels in absence of other mechanical stimuli and soluble gradients. Glioblastoma (U87, HGL21) and breast cancer (MDA-MB-231) cells in 1% agarose hydrogels were exposed to 50% compressive strain for 3 h (0.25–0.05 kPa). Live imaging showed that cells elongate and deflect vertically to the load. This stimulation is shown for the first time to differentially regulate metastasis-associated genes. Furthermore, a group of differentially expressed genes was identified in all cell types, both by microarrays and confirmed by RT-PCR for select genes (caveolin-1, integrin-β1, Rac1), indicating shared response mechanisms. These genes are functionally linked and involved in decreasing cell–cell contact, increasing ECM degradation, and ultimately promoting invasion. Caveolin could orchestrate these responses while the uPA and PI3K/Akt systems could play major roles. Future work will focus on specific molecular partnerships under compression and their impact on cancer progression.
Similar content being viewed by others
Introduction
Mechanotransduction, the conversion of mechanical stimuli to cellular responses, is critical in development, homeostasis, and various diseases.25 Biological effects of mechanical forces have been studied in vitro, in vivo, and computationally for the vasculature, bone, skin, immune system, fetal development, and to a lesser extent tumor cells.6,48 Non-physiological mechanical stimuli arise in tumors in addition to many other dysregulated autocrine and paracrine signals, and gradients of oxygen, pH, and nutrients that generally promote malignancy.16 Specifically, interstitial fluid pressure rises due to peritumoral edema, leaky blood vessels, or malfunctioning lymphatics. Also as tumors grow to the expense of normal tissues, space limitations give rise to non-fluid related pressure (solid stress). This stress depends on tumor size and local mechanical properties, which are influenced by tumor-associated ECM modifications (degradation, crosslinking, overproduction) and chemotherapy drugs that disrupt the cytoskeleton of tumor and host cells. Solid stress is prominent in lowly deformable sites such as cranium and bone causing detrimental morbidity. Intracranial hypertension by brain tumors crushes or modifies brain tissue, and cause hernias, ischemia, or cell death.33 Spinal compression from local tumors interferes with nerves and vessels causing pain, sensory changes, and motor problems.35
Although the pathophysiology of tumor-associated pressure is typically addressed, the impact on the tumor phenotype remains largely unclear. Hydrostatic pressure and other stimuli such as stretch, fluid shear stress, and cell shape- or rigidity-altering chemicals can affect growth, adhesion, or motility of cancer cells (reviewed in Suresh48). In comparison, the effects of non-fluid related pressure are less studied as currently no model exists for applying in vitro solid stress at in vivo-relevant levels. Reasons include the dynamic nature of this stress, deficient instrumentation for measuring it in vivo, and challenges in evaluating its individual biological role amidst complex local phenomena in tumors. To date, effects of non-fluid pressure on cancer cells have been studied by embedding cells or cell clusters in 3D gels of differential stiffness, which was shown to affect cell growth in vitro 22,29 and computationally18,41 and also cell migration.11 ECM stiffness may also modulate epithelial cell fate via integrin signaling leading to neoplasia.24,37
The impact of mechanical compression on the metastatic potential of cancer cells is uncharted territory. To this end, two novel systems, the cell-pressors, were designed to enable molecular analyses and live imaging of 3D cell cultures under compression. Normal mechanical loading is often used in bone studies and is applied by mechanical spectrometers. These machines are most often used in standalone configurations. In some studies mechanical spectrometers have been placed inside cell culture incubators to compress bone cells in 3D gels14 or were attached to cell culture chambers compatible with videomicroscopy.15,27 In these studies, the main goal was to observe morphological changes in chondrocytes under pressure, therefore the culture chamber walls were solid and impermeable to oxygen and nutrients.
The two novel devices described here apply external compression to cells embedded in 3D matrices in the absence of nutrient-, oxygen-, and pH-gradients. The optic-pressor is the first device that enables live cell imaging under externally applied mechanical compression. The well-pressor is also the first device that enables compression of 3D gels in standard commercially available cell culture plates. Both devices are unique in the sense that the load is not applied by a mechanical spectrometer but rather via a manually driven screw attached to a platen. The cell-pressor devices described in this study provide a cost-effective means of compressing cells in 3D and enabled the first ever expression profiling of genes involved in metastasis in absence of other mechanical stimuli or soluble gradients, which could independently affect the tumor phenotype.
Materials and Methods
Cell Culture
Human glioblastoma U87 (ATCC) and HGL21 (Massachusetts General Hospital; Dr. Rakesh Jain) and breast adenocarcinoma MDA-MB-231 (ATCC) cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS, and 1% penicillin/streptomycin (all from Invitrogen) in a humidified 5% CO2–balance air incubator with media renewal every 2 days. At 90% confluency the cells were detached with 2 mM EDTA (Sigma) for passaging or preparation of 3D gels.
Preparation of 3D Tumor Analogs
A stock of 1.5% w/w agarose (type VII-low gelling point, Sigma) in 1× PBS was prepared with autoclaving and addition of sterile water for concentration adjustment. Gels 1% w/w containing 106 cells/mL were prepared in pairs (for compression and corresponding control) by mixing a portion of agarose stock solution with appropriate volume of single-cell suspension in culture media. 1.5 mL of the mixture was either poured in porous inserts located in six-well plates (for use in the well-pressor) or in the chambers of the optic-pressor. The constructs were incubated for 5 min at room temperature followed by 10 min in the cell culture incubator. Then the gels were immersed in culture media and placed in the cell culture incubator for 18 h before exposure to stress.
Design of Custom-Made Cell Compressors
Two custom-made devices, the well-pressor (Fig. 1) and the optic-pressor (Fig. 2), were built for compressing cells embedded in 3D gels aseptically and at physiological temperature and pH. The devices consist of three major parts: the 3D cell culture chamber that contains the cell-populated gel, a pair of an acme screw and nut that applies the pressure, and the housing that supports it. In both devices the gels are surrounded by a precise volume of culture media several folds larger than the gel volume to minimize changes in hydrostatic pressure due to fluid efflux during compression.
In the well-pressor device, the cell-populated gels are placed on commercially available inserts for multi-well culture plates with porous PET membrane base of 8 μm pore size (Fig. 1). Six-well plates and matching inserts (Falcon) were used in this study. The housing for the screw–nut is a poly-methyl-methacrylate (PMMA) cylinder (Fig. 1b). It contains three fixed stainless steel rods that are spaced peripherally and penetrate the flange of the nut to prevent it from rotating along with the screw. The housing is attached on the lid of the multi-well plate with removable screws. The multi-well plate rests snuggly on the ledge of a matching rectangular PMMA base. After the two gels (for compression and control) are prepared for compression (see following section), the lid of the plate, which is already attached to the screw–nut-housing assembly, is positioned on top of the plate and all the pieces (lid–plate-base) are secured together with screw–clamps. The nut is attached to a stainless steel platen that geometrically matches the insert containing the gel with an allowance small enough for free sliding without gel bulging around the platen rim. Therefore, manual rotation of the screw knob moves the nut (which contains a preloaded spring that prevents backlash) linearly toward or away from the gel depending on the direction of rotation. For maintenance of physiological temperature and pH, the well-pressor setup is placed in the cell culture incubator during the experiments.
The optic-pressor has two rectangular parallelepiped chambers that contain the 3D cultures (Fig. 2). The four smaller faces of the chambers are solid PMMA. The two larger faces of the chambers are made of porous PET membranes of 8 μm pore size sandwiched between a stainless steel sieve and a perforated Silastic sheet in contact with the gel. The sieve and the sheet have matching cutouts to create optically clear observation areas throughout the gel. The acme screw–nut assembly is supported by a rectangular PMMA housing that is fastened with screws at the dual culture chamber. During incubation before exposure to stress, the dual chamber of the optic-pressor containing the gels is kept in the cell culture incubator immersed in media inside a Petri dish. For live cell imaging under compression, the fully assembled optic-pressor is submerged in a specific volume of cell culture media inside a custom-made micro-incubator mounted on the microscope stage. The micro-incubator consists of a base and a lid part of optically clear TiO2 glass heated with an external electrical power supply and external temperature control (Omega) to maintain 37 °C in the gel cultures.
To maintain physiological pH, humidified 5% CO2–balance air is infused into the culture media inside the micro-incubator, which surround the gels in the optic-pressor. The CO2–air is humidified by infusion through sterile water in a conical flask. The humidified gas phase of the flask is then infused in the culture media after sterilization through a filter.
Exposure of Cell-Populated 3D Gels to Normal Loading
The devices were sterilized by autoclaving the screws, platens sieves, PET membranes, and UV irradiation of the plastic parts (nuts, housing) prior to each experiment. The plates and inserts of the well-pressor are sterile and disposable. Just before compression, the media were carefully aspirated from the top of the gel to minimize fluid-induced shearing of the cells. The platen was placed on top of the gel. The desired strain was applied by rotating the screw that drives the platen at a predetermined number of rotations so that to impose the same strain level among experiments. 50% strain was applied by half the number of rotations required for moving the platen at a distance equal to the gel thickness. Both systems enable application of precise, compressive load at user-defined levels. The gels were compressed slowly (in order to minimize exposure of the embedded cells to fluid shear stress during media efflux) to 50% of their original volume and were maintained at this state for 3 h. In parallel, controls were prepared with the platen softly touching the gel surface without compressing it. In both systems, the walls of the gel chamber are impermeable to the solid component of the gel (agarose and cells) but allow efflux of water and diffusion of nutrients and oxygen through the PET membrane walls of the chambers, which eliminates gradients and maintains physiological pH throughout the gel. The linearly moving platen imposes a desired step deformation to the gel and transmits a normal force on the embedded cells through the surrounding solid phase without changes in hydrostatic pressure. In this study, the well-pressor was used for gene expression analyses and the optic-pressor for live imaging of cell morphology under stress.
Mechanical testing of the gels was performed with the Dynastat mechanical spectrometer (Dynastatics, Albany, NY, USA) to quantify the stress over time in 1% agarose gels at 50% compressive strain. The platen of the instrument compressed gels inside transwell inserts of 8-μm pore size similar to the layout of the well-pressor setup.
Image Acquisition and Analysis
The NIH ImageJ software was used for acquiring snapshots of live cells in the optic-pressor device and also for measuring cell deflection under normal loading. Images were acquired at different fields of view throughout the 3D matrix. After applying an appropriate threshold (for cell selection) the images were binarized and ellipses were fitted to simulate the shape of cells (using standard functions of the software). The elliptical shape simulated well the rounded morphology of the cells in agarose gels. Cell deflection under stress was quantified in terms of changes in cell circularity and orientation. Specifically, the “Fit Ellipse” function extracted the lengths of the major and minor axes of the ellipses (cells). The “Circularity” function, which calculates the ratio of the minor axis vs. the major axis, was used to quantify the deformation of the cells under compression. The angle that the major axis of the ellipses (cells) formed with the direction of the applied force was used to quantify the change of cell orientation under loading.
RNA Isolation and Quality Testing
After 3 h exposure to 50% compressive strain the gels were removed without decompression by dismantling the devices. Total RNA was purified with the TRIzol® reagent (Invitrogen) according to manufacturer’s protocol. During purification a high salt solution (0.8 M sodium citrate and 1.2 M NaCl) was added to the aqueous phase to prevent coprecipitation of agarose with the RNA. Before all gene expression assays good RNA quality was assured spectroscopically (OD260/280 > 1.8) and by intact ribosomal bands in diagnostic electrophoresis gels.
Gene Expression Profiling
Gene expression was assayed with function-specific microarrays containing oligos of human genes associated with metastasis (SuperArray) as described previously.12 Each array contained 113 genes, housekeeping genes, and negative/positive hybridization control spots. The probe was synthesized from 1 μg total RNA using the Amp2 kit (SuperArray) according to manufacturer’s instructions. The chemiluminescence signal of the arrays was captured in radiographic films that were subsequently scanned to generate digital images. These images were analyzed to extract the gray value of each gene spot utilizing a custom-made mosaic of regions of interest constructed with the NIH ImageJ software (http://rsb.info.nih.gov/ij/). Relative gene expression in each array was quantified by subtracting the gray value of the background (average gray value of negative spots) from the gray value corresponding to each gene spot and then normalizing by dividing with the average gray value of the housekeeping genes. Two independent sets of compression/control experiments (independent RNA samples and array membranes) were performed for each cell line.
Taqman RT-PCR
Expression of caveolin 1, integrin β1, and Rac1 was confirmed with real-time PCR utilizing the Taqman technology and the AB7500 Fast Real-Time PCR System (Applied Biosystems), starting with 150 ng DNase-treated total RNA. Three replicates per experimental condition were performed. C T values were extracted with the SDS software (Applied Biosystems) and relative gene expression analysis was based on the ΔΔC T method, using the expression of GAPDH as control.
Results
Custom-Made Cell Compressors
Cancer cells embedded in 3D gels were exposed to normal mechanical force in two novel custom-made devices as an in vitro model of solid stress in tumors. The well-pressor (Fig. 1) is attached to multi-well plates for compressing gels inside transwell filters. The optic-pressor (Fig. 2) fits in a custom-made micro-incubator on the microscope stage for live cell imaging under compression. Both devices apply confined compressive strain to the solid component of the gel (and cells) and allow efflux of water under compression and free diffusion of nutrients and oxygen through the filter walls. Water efflux from a gel increases the concentration and potentially the propensity for cell–ECM bonding/interaction, which can affect cytoskeleton-mediated signaling. Therefore, agarose, an inert polysaccharide material, was utilized in this study for its small cell-affinity and in order to specifically assess the effect of compression on the metastatic potential of cancer cells. The devices utilize inexpensive consumable or easily sterilized materials, and provide a cost-effective means of applying a range of compressive strain level by adjusting the compressive strain and/or the concentration of the 3D matrix. The stress level inside the gel depends on the vol.% compression and the concentration of the agarose gel.
Stress vs. time for 1% w/w agarose hydrogels exposed to 50% compressive strain is plotted in Fig. 3. Maximum stress of 0.25 kPa occurs immediately after applying the external load and is subsequently decreased over 3 h to the plateau level of 0.05 kPa as the solid component of the gel gradually compacts.
Cell Imaging Under Compressive Strain
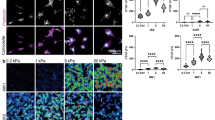
The optic-pressor enables 3D live imaging of the cells under normal loading (Fig. 4) and quantification of cell deformation, which is a major mediator of gene transcription and other cell functions.48 In agarose gels (an inert matrix) the cells had a rounded morphology. When the compression was applied, they obtained an oval morphology and aligned perpendicular to the direction of the applied force. The cell deflection was quantified here through changes in cell circularity and cell orientation with respect to the direction of the applied force (Figs. 4 and 5) immediately after the application of strain. More specifically, circularity changed from 0.852 (mean) ± 0.035 (standard deviation) to 0.810 ± 0.048. The angle of cell orientation changed from 63.1° (mean) ± 50.66 (standard deviation) to 87.96° ± 6.68. One-way ANOVA test concluded that the changes were statistically significant at the levels shown in Fig. 5. Similar shape changes were observed previously for chondrocytes compressed in agarose gels.4,28,32
(a) Phase contrast imaging of U87 cells in 1% agarose hydrogels inside the optic-pressor device before application of compressive strain. (b) Outlines of cells labeled “1,” “2,” and “3” and their orientation with respect to axis X. (c) Deformation of cells (from panel “a”) under externally applied compression in the direction of axis X. (d) Under external loading the cells align perpendicular to the direction of the applied force. The relative position of panels (b) and (d) is marked in the raw images (a) and (c), respectively
Boxplots for (a) cell circularity (ratio of the minor axis vs. major axis) and (b) cell orientation (angle between the major axis and the direction of the applied force) for the fitted ellipses simulating the cell shape for control (uncompressed) cells and immediately after the application of 50% compressive strain. The plots show the max, min, median, 25th, and 75th percentiles of the distribution. The “*” denotes statistically significant differences determined by one-way ANOVA (p-value shown; the plots correspond to the analysis of 40 cells in 7 fields of view)
Compressed Cancer Cells Express Differentially Metastasis-Associated Genes
In order to quantify the effect of mechanical compression on the potential of cancer cells to metastasize, differential expression of genes involved in metastasis was quantified in cells exposed to normal loading vs. control cells. This analysis was performed for two lines of glioblastoma U87 and HGL21 and the MDA-MB-231 breast cancer cells. These cells were chosen for this study on the basis that solid stress could be a significant factor in the mechanobiology of tumors growing in the confined space of the scull or in the bone. Glioblastoma are brain tumors and human breast cancer has a high rate of metastasis in the brain and bone. Table 1 summarizes the genes expressed differentially in all cell types and real-time PCR confirmation for the select genes caveolin 1, integrin β1, and Rac1. Genes highlighted in color were differentially expressed in common by two (blue) or three (yellow) cell types.
Discussion
For the first time expression profiles of metastasis-associated genes were quantified for tumor cells in 3D cultures exposed to compressive strain. The level of 50% compressive strain of 1% agarose gels was chosen as a sufficient pressure for generating a visible change in cell shape (which can be observed microscopically with the optic-pressor) and also for inducing differential gene expression without causing any apparent cell damage (as deduced by observing the cells under the microscope and from similar RNA yield from compressed and control gels, which contain same number of cells). Exposure to mechanical stimuli for 3 h is a common practice in previously published studies of cells exposed to mechanical stimulation. In this study, 3 h exposure to stress was chosen for being sufficient to induce differential gene expression and demonstrate proof of concept for the effects of mechanical compression without significant cell damage/death. Tumor cells were also exposed to normal loading for 6 and 12 h at the well-pressor. In these cases differential gene expression was also observed although with different gene profiles compared to the 3-h exposure (data not shown). The dependence of differential gene expression on the level and duration of a particular mechanical stimulus (for a specific cell type) is well established by many previous mechanotransduction studies. Dose–response studies in the future will investigate the effect of exposure time on cancer cell phenotype (including metastatic potential and cell death/apoptosis) under normal loading.
The stress applied (0.05–0.25 kPa), corresponding to 50% compressive strain in 1% agarose gels, was at least one order of magnitude smaller than typical intracranial hypertension and two orders smaller than normal loads that induce mechanotransduction in bone cells.39 Although exact levels of solid stress have not been measured yet in actual tumors, the stress in this study was applied in a level smaller than measured in vivo stresses that are known for playing a role in tumor phenotype (e.g., intracranial pressure) or induce differential gene expression (e.g., in bone cells). Solid stress in vivo stems from the crowding of tumor cells; in this study, the gels were populated by single cells at a relatively low seeding density. This allows quantification of the differential effect of mechanical stress in gene expression in absence of other stresses or biological stimuli that can also stem from the crowding of tumor cells such as hypoxia, pH, and nutrient gradients, which are known to affect the phenotype of tumor cells.
During compression for 3 h, formation of oxygen or nutrient gradients in the gels is expected to be insignificant for the following reasons: media were incorporated in the 3D matrices during making and they were subsequently incubated surrounded by culture media overnight before exposure to stress; the matrices were only a couple of millimeters thick and during exposure to stress/control were in contact with cell culture media through porous filters and in a controlled CO2 atmosphere; the cell seeding density was relatively low, and 3 h is a relatively short exposure time compared to the time typically required for cell metabolism. In addition, compressed specimens and controls are positioned in identical experimental setups thus effects of any potential gradients on differential gene expression should be eliminated at large. However, for longer exposure periods the system should be characterized in detail in terms of formation of oxygen and nutrient gradients.
Although the cell-pressors lack the dynamic and computerized loading capabilities of mechanical spectrometers, they provide a cost-effective alternative for applying step strain to 3D gels with their low manufacturing and operating cost compared to spectrometers. Moreover, the advantage of the two novel cell-pressor devices is the ability to apply mechanical pressure to cell-populated 3D matrices in absence of nutrient-, oxygen-, and pH-gradients. This exclusive mechanical stimulation is particularly important for studying in vitro solid stress since the abovementioned stimuli independently affect the phenotype of tumor cells. Furthermore, the optic-pressor is the first ever device that is videomicroscopy compatible and enables live imaging of cells in 3D gels under normal loading in absence of nutrient-, oxygen-, and pH-gradients.
Despite cell line-specific individualities, a shared set of genes was differentially expressed in three independent cell lines suggesting that cancer cells may respond to compression via shared mechanisms. Whether the genes are individually regulated by stress or respond to down-stream signaling remains to be investigated. However, identifying force–response elements has been difficult even for better-studied mechanical stimuli such as fluid shear.54 Individual and synergistic functions of the genes are discussed below.
Compression May Promote Local ECM Proteolysis, Cell Adhesion, and Migration
Potential Orchestrating Role of Caveolin 1
Cav1, the main component of the membrane invaginations caveolae, could play a center stage role in the phenotype of compressed tumor cells (Fig. 6). To begin with, cav1 could be involved in “sensing” compression (Fig. 6a) similar to its role as shear stress mechanosensor in endothelial cells.9 Interestingly, other endothelial cell mechanosensors such as cadherin 1 (E-cadherin, CDH1) and PECAM-1 (CD31)9 were down-expressed in this study. In general, the roles of cav1 in cancer remain controversial and tissue-specific.38 Although considered a tumor suppressor for maintaining pro-proliferative and pro-survival proteins of the PI3K/Akt pathway in inactive states,47 cav1 can also promote tumor cell survival and metastasis.3,42 Specifically, many differentially expressed molecules in this study localize in caveolae and interact to promote proteolysis, invasion, and migration such as cathepsin B, MMP2, MMP14, TIMP2, integrins, CD44, and uPAR.2,7,45
Potential roles of caveolin 1 in the tumor phenotype under compression. Compression “sensed” via cav1 (a) or other mechanosensors (b) induced differential upregulation of cav1 which could modify local signaling and ultimately affect adhesion, migration, and invasion (c). In addition, various molecules that were also differentially overexpressed can localize at caveolae and form partnerships that enhance proteolysis, adhesion, migration, and invasion via: recruitment and activation of MMPs and cathepsins (d), involvement of the uPA system (e), or integrin-mediated pathways such as Src, FAK, Rac-PAK, Ras-Erk, and PI3k/Akt (f). In particular, many genes are involved in the PI3K/Akt pathway that controls cell cycle (g). In addition, CD44 localized in caveolae at the invasive cell front could facilitate recruitment of MMPs and signaling molecules that interact with the cytoskeleton ultimately enhancing migration and invasion (h)
Local Proteolysis
uPA, uPAR, cathepsins, and MMPs form proteolytic partnerships in caveolae and are predictors of poor cancer prognosis. uPA and uPAR play major roles in adhesion, motility, intravasation, invasion, and proliferation of many types of cancer including brain and breast.49 uPA converts plasminogen to plasmin that degrades ECM or activates other proteases such as MMPs. Activation of the uPA system by compressive mechanical stress also occurs in endothelial, epithelial, and bone cells.8,34 Cathespin-B, which degrades ECM directly or activates other proteases, localizes at the invasive front of brain and adenocarcinoma tumors and is linked to tumor growth, invasion, and angiogenesis.46 In caveolae, cathepsin B activates soluble or membrane-bound pro-uPA while reciprocally pro-cathepsin B is activated by uPA or cathepsin D (overexpressed here in MDA-MB-231 cells). TIMP2 (overexpressed in MDA-MB-231) binds to MMP14 and activates pro-MMP22 (MMP2 being critical in breast cancer metastasis).
Adhesion and Migration
Molecules that decrease intercellular bonding increasing motility and invasion were downregulated by compression. Specifically, downregulation of CDH1, which forms the core of adherens junctions at cell–cell contact sites, generally promotes cancer progression.53 Also PTEN, which decreases CDH1-mediated cell–cell adhesion,52 was overexpressed. Rac1, which was overexpressed in all cell lines, is critical in cancer cell migration56 and increases motility and invasion when CDH1-mediated cell–cell adhesion is decreased.23
In addition, members of the integrin family of cell adhesion molecules were overexpressed. Mechanical forces typically upregulate integrins, which are major mechanoreceptors, reinforce linkage to the ECM, and increase motility and invasion.20,24,48,57 ITGβ1, which was highly overexpressed in all cell types, is critical in tumorigenesis and maintenance of the proliferative capacity of cancer cells.55 Combined upregulation of ITGα3 and β1 (as shown here) was also observed previously under hydrostatic pressure.21 In general, cancer cells regulate integrins in response to environmental stimuli for modulating survival, migration, proliferation, invasion, or metastasis.20 Partnerships involving cav1 could regulate cell polarization and directional migration of compressed tumor cells via major integrin-mediated pathways such as Rac-PAK, Ras-Erk, and PI3k/Akt10,19 (Fig. 6e). Normal function of ITGβ1 requires cav1 and is regulated by uPAR 43 via a uPAR–integrin–cav1 complex (Fig. 6e), which was previously identified at the adhesion sites of carcinoma cells.3
Interestingly, upregulation of CD44 could significantly impact invasion of brain tumors and breast cancer metastases considering that CD44 is the major receptor of hyaluronic acid (HA), the main component of the central nervous system (CNS)1; compressive forces are prevalent in brain tumors and bones; and breast cancer typically metastasizes at high rates to the CNS and bone. CD44 is known to localize at the invasive front of aggressive glioblastomas and carcinomas; participates in signaling, recruitment of membrane receptors, and MMPs; and interacts with the cytoskeleton to promote migration and invasion26 (Fig. 6d). HA recruits CD44 into caveolae and interacts with the underlying actin cytoskeleton to activate Rac1 45 and cause lamellipodia outgrowth36 (Fig. 6h). Moreover, cancer cells in response to HA upregulate molecules involved in proteolysis such as uPA, uPAR, plasminogen activator inhibitor-1 (PAI-1), and TIMP - 1.51
Potential Roles of Tumor Suppressors
Genes typically referred to as negative tumor regulators were also upregulated under compression. Specifically, cav1, PTEN, caspase, CSF-1, and CDH1 are associated with the PI3K/Akt pathway (Fig. 6g) that controls cell cycle and apoptosis and is critical in cancer progression.13 In addition, cav1 regulates cell growth via the Rac-PAK or Ras-Erk pathway.10 PTEN, a negative regulator of PI3K-Akt signaling (CSF-1 lies upstream of PTEN), is often inactivated in advanced cancers and inhibits cell spreading and motility in a tissue-specific manner.13 Although solid stress could inhibit tumor growth or induce apoptosis22 its roles could be far more complex and dependent on the stress level (or tumor size). Considering that mechanical stress naturally arises in tumors solid stress could be a mechanism “exploited” by tumors to facilitate their progression. Interestingly, cav1, caspase 8, PTEN, and FGF-2 could interact with other differentially expressed genes under compression to ultimately stretch their function as negative tumor regulators and promote cancer progression by:
-
Promoting growth or survival: Specifically, autocrine or paracrine (in response to tissue damage) cav1 suppressed apoptosis and increased proliferation of prostate cells.3 Increased expression of PECAM-1 in MDA-MB-231 cells caused loss of CD44 and growth reduction.40 In this study an inverse correlation was identified with CD44 being upregulated and PECAM-1 downregulated. In another model breast cancer cells survived in the bone marrow by FGF-2-mediated growth arrest and upregulation of integrin α5β1 (FGF-2 and ITGβ1 overexpressed here for MDA-MB-231). This inhibited apoptosis and promoted viability via binding to fibronectin while proliferative capacity was restored upon removal of FGF-2 and involvement of the PI3k/Akt pathway.30 This dormancy mechanism could contribute to the phenotype of growing tumors since growth causes tissue damage that increases the local levels of fibronectin50 and also mechanical stress, which is shown here to differentially regulate implicated genes.
-
Regulating directional migration: Upregulation of PTEN could help direct cell migration under compression.52PTEN localizes at the leading edge of migrating cells,17 stabilizes CDH1-mediated cell–cell adhesion,31 or downregulates uPA.44 Finally, despite its role in apoptosis, caspase 8 is also required for uPA-dependent motility and invasion5 (both genes upregulated in U87 cells).
Conclusions
This study demonstrates for the first time that normal loading could regulate expression of genes involved in ECM degradation, cell–cell contact, migration, and proliferation. Therefore, mechanical compression (a common phenomenon in tumors) could promote invasion and metastasis independently of other non-physiological mechanobiological stimuli in the tumor microenvironment. In the future the cell-pressor devices will help investigate molecular partnerships under compression and their impact on the cancer phenotype and progression.
References
Akiyama, Y., S. Jung, B. Salhia, S. Lee, S. Hubbard, M. Taylor, T. Mainprize, K. Akaishi, W. Van Furth, and J. T. Rutka. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neuro-Oncol. 53:115–127, 2001.
Annabi, B., M. Lachambre, N. Bousquet-Gagnon, M. Page, D. Gingras, and R. Beliveau. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem. J. 353:547–553, 2001.
Ayala, G. E., H. Dai, S. A. Tahir, R. Li, T. Timme, M. Ittmann, A. Frolov, T. M. Wheeler, D. Rowley, and T. C. Thompson. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 66:5159–5164, 2006.
Bader, D. L., T. Ohashi, M. M. Knight, D. A. Lee, and M. Sato. Deformation properties of articular chondrocytes: a critique of three separate techniques. Biorheology 39:69–78, 2002.
Barnhart, B. C., P. Legembre, E. Pietras, C. Bubici, G. Franzoso, and M. E. Peter. Cd95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 23:3175–3185, 2004.
Brown, T. D. Techniques for mechanical stimulation of cells in vitro: a review. J. Biomech. 33:3–14, 2000.
Cavallo-Medved, D., J. Mai, J. Dosescu, M. Sameni, and B. F. Sloane. Caveolin-1 mediates the expression and localization of cathepsin b, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J. Cell Sci. 118:1493–1503, 2005.
Chu, E. K., J. Cheng, J. S. Foley, B. H. Mecham, C. A. Owen, K. J. Haley, T. J. Mariani, I. S. Kohane, D. J. Tschumperlin, and J. M. Drazen. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 35:628–638, 2006.
Davies, P. F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 6:16–26, 2009.
Del Pozo, M. A., and M. A. Schwartz. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 17:246–250, 2007.
Demou, Z. N., M. Awad, T. Mckee, J. Y. Perentes, X. Wang, L. L. Munn, R. K. Jain, and Y. Boucher. Lack of telopeptides in fibrillar collagen I promotes the invasion of a metastatic breast tumor cell line. Cancer Res. 65:5674–5682, 2005.
Demou, Z. N., and M. J. C. Hendrix. Microgenomics profile the endogenous angiogenic phenotype in subpopulations of aggressive melanoma. J. Cell Biochem. 105:562–573, 2008.
Dillon, R. L., D. E. White, and W. J. Muller. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26:1338–1345, 2007.
Frank, E. H., M. Jin, A. M. Loening, M. E. Levenston, and A. J. Grodzinsky. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J. Biomech. 33:1523–1527, 2000.
Freeman, P. M., R. N. Natarajan, J. H. Kimura, and T. P. Andriacchi. Chondrocyte cells respond mechanically to compressive loads. J. Orthop. Res. 12:311–320, 1994.
Fukumura, D., and R. K. Jain. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J. Cell. Biochem. 101:937–949, 2007.
Goberdhan, D. C., and C. Wilson. Pten: tumour suppressor, multifunctional growth regulator and more. Hum. Mol. Genet. 12(2):R239–R248, 2003.
Gordon, V. D., M. T. Valentine, M. L. Gardel, D. Andor-Ardo, S. Dennison, A. A. Bogdanov, D. A. Weitz, and T. S. Deisboeck. Measuring the mechanical stress induced by an expanding multicellular tumor system: a case study. Exp. Cell Res. 289:58–66, 2003.
Grande-Garcia, A., A. Echarri, J. De Rooij, N. B. Alderson, C. M. Waterman-Storer, J. M. Valdivielso, and M. A. Del Pozo. Caveolin-1 regulates cell polarization and directional migration through src kinase and rho gtpases. J. Cell Biol. 177:683–694, 2007.
Guo, W., and F. G. Giancotti. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5:816–826, 2004.
Haskin, C., I. Cameron, and K. Athanasiou. Physiological levels of hydrostatic pressure alter morphology and organization of cytoskeletal and adhesion proteins in mg-63 osteosarcoma cells. [erratum appears in Biochem. Cell Biol. 1993 May–June; 71(5–6):313]. Biochem. Cell Biol. 71:27–35, 1993.
Helmlinger, G., P. A. Netti, H. C. Lichtenbeld, R. J. Melder, and R. K. Jain. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15:778–783, 1997.
Hu, L., W. Hittelman, T. Lu, P. Ji, R. Arlinghaus, I. Shmulevich, S. R. Hamilton, and W. Zhang. Ngal decreases e-cadherin-mediated cell-cell adhesion and increases cell motility and invasion through rac1 in colon carcinoma cells. Lab Invest. 89(5):531–548, 2009.
Huang, S., and D. E. Ingber. Cell tension, matrix mechanics, and cancer development. Cancer Cell 8:175–176, 2005.
Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20:811–827, 2006.
Jothy, S. Cd44 and its partners in metastasis. Clin. Exp. Metastasis 20:195–201, 2003.
Knight, M. M., S. A. Ghori, D. A. Lee, and D. L. Bader. Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Med. Eng. Phys. 20:684–688, 1998.
Knight, M. M., D. A. Lee, and D. L. Bader. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim. Biophys. Acta 1405:67–77, 1998.
Koike, C., T. D. Mckee, A. Pluen, S. Ramanujan, K. Burton, L. L. Munn, Y. Boucher, and R. K. Jain. Solid stress facilitates spheroid formation: potential involvement of hyaluronan. Br. J. Cancer 86:947–953, 2002.
Korah, R., M. Boots, and R. Wieder. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 64:4514–4522, 2004.
Kotelevets, L., J. Van Hengel, E. Bruyneel, M. Mareel, F. Van Roy, and E. Chastre. The lipid phosphatase activity of pten is critical for stabilizing intercellular junctions and reverting invasiveness. J. Cell Biol. 155:1129–1135, 2001.
Lee, D. A., T. Noguchi, M. M. Knight, L. O’donnell, G. Bentley, and D. L. Bader. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J. Orthop. Res. 16:726–733, 1998.
Marmarou, A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 22:1E, 2007.
Mitsui, N., N. Suzuki, Y. Koyama, M. Yanagisawa, K. Otsuka, N. Shimizu, and M. Maeno. Effect of compressive force on the expression of mmps, pas, and their inhibitors in osteoblastic saos-2 cells. Life Sci. 79:575–583, 2006.
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2:584–593, 2002.
Oliferenko, S., I. Kaverina, J. V. Small, and L. A. Huber. Hyaluronic acid (ha) binding to cd44 activates rac1 and induces lamellipodia outgrowth. [erratum appears in J. Cell Biol. 2000 Apr 3;149(1):Following 236]. J. Cell Biol. 148:1159–1164, 2000.
Paszek, M. J., N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, D. A. Hammer, and V. M. Weaver. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8:241–254, 2005.
Prinetti, A., S. Prioni, N. Loberto, M. Aureli, V. Chigorno, and S. Sonnino. Regulation of tumor phenotypes by caveolin-1 and sphingolipid-controlled membrane signaling complexes. Biochim. Biophys. Acta 1780:585–596, 2008.
Rath, B., J. Nam, T. J. Knobloch, J. J. Lannutti, and S. Agarwal. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech. 41:1095–1103, 2008.
Righi, L., S. Deaglio, C. Pecchioni, A. Gregorini, A. L. Horenstein, G. Bussolati, A. Sapino, and F. Malavasi. Role of cd31/platelet endothelial cell adhesion molecule-1 expression in in vitro and in vivo growth and differentiation of human breast cancer cells. Am. J. Pathol. 162:1163–1174, 2003.
Roose, T., P. A. Netti, L. L. Munn, Y. Boucher, and R. K. Jain. Solid stress generated by spheroid growth estimated using a linear poroelasticity model small star, filled. Microvasc. Res. 66:204–212, 2003.
Satoh, T., G. Yang, S. Egawa, J. Addai, A. Frolov, S. Kuwao, T. L. Timme, S. Baba, and T. C. Thompson. Caveolin-1 expression is a predictor of recurrence-free survival in pt2n0 prostate carcinoma diagnosed in Japanese patients. Cancer 97:1225–1233, 2003.
Schwab, W., J. M. Gavlik, T. Beichler, R. H. Funk, S. Albrecht, V. Magdolen, T. Luther, M. Kasper, and M. Shakibaei. Expression of the urokinase-type plasminogen activator receptor in human articular chondrocytes: association with caveolin and beta 1-integrin. Histochem. Cell Biol. 115:317–323, 2001.
Shukla, S., G. T. Maclennan, D. J. Hartman, P. Fu, M. I. Resnick, and S. Gupta. Activation of pi3k-akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 121:1424–1432, 2007.
Singleton, P. A., R. Salgia, L. Moreno-Vinasco, J. Moitra, S. Sammani, T. Mirzapoiazova, and J. G. Garcia. Cd44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-met, tiam1/rac1, dynamin 2, and cortactin. J. Biol. Chem. 282:30643–30657, 2007.
Skrzydlewska, E., M. Sulkowska, M. Koda, and S. Sulkowski. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J. Gastroenterol. 11:1251–1266, 2005.
Sotgia, F., H. Rui, G. Bonuccelli, I. Mercier, R. G. Pestell, and M. P. Lisanti. Caveolin-1, mammary stem cells and estrogen-dependent breast cancers. Cancer Res. 66:10647–10651, 2006.
Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 3:413–438, 2007.
Tang, C. H., and Y. Wei. The urokinase receptor and integrins in cancer progression. Cell. Mol. Life Sci. 65:1916–1932, 2008.
Tate, C. C., M. C. Tate, and M. C. Laplaca. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J. Neurotrauma 24:226–230, 2007.
Tsatas, D., V. Kanagasundaram, A. Kaye, and U. Novak. Egf receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J. Clin. Neurosci. 9:282–288, 2002.
Vogelmann, R., M. D. Nguyen-Tat, K. Giehl, G. Adler, D. Wedlich, and A. Menke. Tgfbeta-induced downregulation of e-cadherin-based cell-cell adhesion depends on pi3-kinase and pten. J. Cell Sci. 118:4901–4912, 2005.
Wells, A., C. Yates, and C. R. Shepard. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin. Exp. Metastasis 25:621–628, 2008.
White, C. R., and J. A. Frangos. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 362:1459–1467, 2007.
White, D. E., N. A. Kurpios, D. Zuo, J. A. Hassell, S. Blaess, U. Mueller, and W. J. Muller. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6:159–170, 2004.
Yamazaki, D., S. Kurisu, and T. Takenawa. Involvement of rac and rho signaling in cancer cell motility in 3d substrates. Oncogene 28:1570–1583, 2009.
Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. Effects of substrate stiffness on cell morphology, cytoskeletal structure and adhesion. Cell Motil. Cytoskeleton 60:24–34, 2005.
Acknowledgments
Part of this study was performed at the Massachusetts General Hospital with support of a postdoctoral grant to Z.N.D. from the Susan G. Komen Breast Cancer Foundation. The author thanks Drs. Rakesh Jain and Lance Munn and also Dr. Eliot H. Frank for his expert help with the mechanical testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott Simon oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Demou, Z.N. Gene Expression Profiles in 3D Tumor Analogs Indicate Compressive Strain Differentially Enhances Metastatic Potential. Ann Biomed Eng 38, 3509–3520 (2010). https://doi.org/10.1007/s10439-010-0097-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0097-0