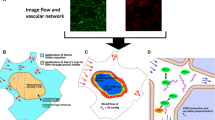
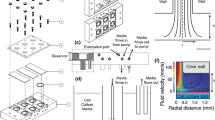
Abstract
Convective transport can significantly distort spatial concentration gradients. Interstitial flow is ubiquitous throughout living tissue, but our understanding of how interstitial flow affects concentration gradients in biological processes is limited. Interstitial flow is of particular interest for angiogenesis because pathological and physiological angiogenesis is associated with altered interstitial flow, and both interstitial flow and morphogen gradients (e.g., vascular endothelial growth factor, VEGF) can potentially stimulate and guide new blood vessel growth. We designed an in vitro microfluidic platform to simulate 3D angiogenesis in a tissue microenvironment that precisely controls interstitial flow and spatial morphogen gradients. The microvascular tissue was developed from endothelial colony forming cell-derived endothelial cells extracted from cord blood and stromal fibroblasts in a fibrin extracellular matrix. Pressure in the microfluidic lines was manipulated to control the interstitial flow. A mathematical model of mass and momentum transport, and experimental studies with fluorescently labeled dextran were performed to validate the platform. Our data demonstrate that at physiological interstitial flow (0.1–10 μm/s), morphogen gradients were eliminated within hours, and angiogenesis demonstrated a striking bias in the opposite direction of interstitial flow. The interstitial flow-directed angiogenesis was dependent on the presence of VEGF, and the effect was mediated by αvβ3 integrin. We conclude that under physiological conditions, growth factors such as VEGF and fluid forces work together to initiate and spatially guide angiogenesis.





Similar content being viewed by others
References
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257. doi:10.1038/35025220
Khurana R, Simons M, Martin JF, Zachary IC (2005) Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112(12):1813–1824. doi:10.1161/CIRCULATIONAHA.105.535294
Swartz MA, Lund AW (2012) Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 12(3):210–219. doi:10.1038/nrc3186
Roussos ET, Condeelis JS, Patsialou A (2011) Chemotaxis in cancer. Nat Rev Cancer 11(8):573–587. doi:10.1038/nrc3078
Wiig H, Swartz MA (2012) Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92(3):1005–1060. doi:10.1152/physrev.00037.2011
Song JW, Munn LL (2011) Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA 108(37):15342–15347. doi:10.1073/pnas.1105316108
Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD (2014) Mechanotransduction of fluid stresses governs 3D cell migration. Proc Natl Acad Sci USA 111(7):2447–2452. doi:10.1073/pnas.1316848111
Shi ZD, Tarbell JM (2011) Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng 39(6):1608–1619. doi:10.1007/s10439-011-0309-2
Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13(12):871–882. doi:10.1038/nrc3627
Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10(2):116–129. doi:10.1038/nrc2780
Jain RK, Schlenger K, Hockel M, Yuan F (1997) Quantitative angiogenesis assays: progress and problems. Nat Med 3(11):1203–1208
Bischel LL, Young EW, Mader BR, Beebe DJ (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34(5):1471–1477. doi:10.1016/j.biomaterials.2012.11.005
Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109(24):9342–9347. doi:10.1073/pnas.1201240109
Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA (2007) Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11(6):526–538. doi:10.1016/j.ccr.2007.04.020
Alonzo LF, Moya ML, Shirure VS, George SC (2015) Microfluidic device to control interstitial flow-mediated homotypic and heterotypic cellular communication. Lab Chip 15(17):3521–3529
Hsu YH, Moya ML, Abiri P, Hughes CC, George SC, Lee AP (2013) Full range physiological mass transport control in 3D tissue cultures. Lab Chip 13(1):81–89. doi:10.1039/c2lc40787f
Moya ML, Hsu YH, Lee AP, Hughes CC, George SC (2013) In vitro perfused human capillary networks. Tissue Eng Part C Methods 19(9):730–737. doi:10.1089/ten.TEC.2012.0430
Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J (2007) In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109(11):4761–4768. doi:10.1182/blood-2006-12-062471
Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC (2010) Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A 16(2):585–594. doi:10.1089/ten.TEA.2009.0491
Luckett PM, Fischbarg J, Bhattacharya J, Silverstein SC (1989) Hydraulic conductivity of endothelial cell monolayers cultured on human amnion. Am J Physiol 256(6 Pt 2):H1675–H1683
Nuttall AL (1987) Velocity of red blood cell flow in capillaries of the guinea pig cochlea. Hear Res 27(2):121–128
Ivanov KP, Kalinina MK, Levkovich YuI (1981) Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc Res 22(2):143–155
Swartz MA, Fleury ME (2007) Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng 9:229–256. doi:10.1146/annurev.bioeng.9.060906.151850
Jain RK, Stock RJ, Chary SR, Rueter M (1990) Convection and diffusion measurements using fluorescence recovery after photobleaching and video image analysis: in vitro calibration and assessment. Microvasc Res 39(1):77–93
Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA (2013) Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA 110(12):4563–4568. doi:10.1073/pnas.1221602110
Rutkowski JM, Swartz MA (2007) A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol 17(1):44–50. doi:10.1016/j.tcb.2006.11.007
Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ (2006) Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng 12(10):2875–2888. doi:10.1089/ten.2006.12.2875
Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, Putnam AJ, George SC (2008) The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J 94(5):1930–1941. doi:10.1529/biophysj.107.120774
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264(5158):569–571
MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, Laug WE (2001) Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery 48(1):151–157
Liu Z, Wang F, Chen X (2008) Integrin alpha(v)beta(3)-targeted cancer therapy. Drug Dev Res 69(6):329–339. doi:10.1002/ddr.20265
Chen J, Green J, Yurdagul A Jr, Albert P, McInnis MC, Orr AW (2015) alphavbeta3 integrins mediate flow-induced NF-kappaB activation, proinflammatory gene expression, and early atherogenic inflammation. Am J Pathol 185(9):2575–2589. doi:10.1016/j.ajpath.2015.05.013
Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS (2003) alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 6(2):105–119. doi:10.1023/B:AGEN.0000011801.98187.f2
Hompland T, Ellingsen C, Ovrebo KM, Rofstad EK (2012) Interstitial fluid pressure and associated lymph node metastasis revealed in tumors by dynamic contrast-enhanced MRI. Cancer Res 72(19):4899–4908. doi:10.1158/0008-5472.CAN-12-0903
Chary SR, Jain RK (1989) Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci USA 86(14):5385–5389
Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, Sudo R, Kamm RD, Chung S (2011) In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11(13):2175–2181. doi:10.1039/c1lc20039a
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6):1163–1177. doi:10.1083/jcb.200302047
Gerhardt H (2008) VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4(4):241–246
Kim S, Lee H, Chung M, Jeon NL (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13(8):1489–1500. doi:10.1039/c3lc41320a
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC (2011) The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22(20):3791–3800. doi:10.1091/mbc.E11-05-0393
Lee H, Kim S, Chung M, Kim JH, Jeon NL (2014) A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res 91:90–98. doi:10.1016/j.mvr.2013.12.001
Sobrino A, Phan DT, Datta R, Wang X, Hachey SJ, Romero-Lopez M, Gratton E, Lee AP, George SC, Hughes CC (2016) 3D microtumors in vitro supported by perfused vascular networks. Sci Rep 6:31589. doi:10.1038/srep31589
Phan DT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LY, George SC, Lee AP, Hughes CC (2017) A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 17(3):511–520. doi:10.1039/c6lc01422d
Sobrino A, Phan DT, Wang X, Datta R, Hachey SJ, López MR, Gratton E, Lee AP, George SC, Hughes CCW (2016) A vascularized and perfused human micro-tumor platform for cancer drug screening. SC Rep (in press)
Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A (2006) Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 98(5):335–344. doi:10.1093/jnci/djj070
Nagy JA, Feng D, Vasile E, Wong WH, Shih SC, Dvorak AM, Dvorak HF (2006) Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab Invest 86(8):767–780. doi:10.1038/labinvest.3700436
Vickerman V, Blundo J, Chung S, Kamm R (2008) Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8(9):1468–1477. doi:10.1039/b802395f
Verbridge SS, Chakrabarti A, DelNero P, Kwee B, Varner JD, Stroock AD, Fischbach C (2013) Physicochemical regulation of endothelial sprouting in a 3D microfluidic angiogenesis model. J Biomed Mater Res A 101(10):2948–2956. doi:10.1002/jbm.a.34587
Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS (2013) Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci USA 110(17):6712–6717. doi:10.1073/pnas.1221526110
Chan JM, Zervantonakis IK, Rimchala T, Polacheck WJ, Whisler J, Kamm RD (2012) Engineering of in vitro 3D capillary beds by self-directed angiogenic sprouting. PLoS ONE 7(12):e50582. doi:10.1371/journal.pone.0050582
Li L, Wang L, Zhang W, Tang B, Zhang J, Song H, Yao D, Tang Y, Chen X, Yang Z, Wang G, Li X, Zhao J, Ding H, Reed E, Li QQ (2004) Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res 24(3b):1973–1979
Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS (2014) Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci USA 111(22):7968–7973. doi:10.1073/pnas.1310842111
Vickerman V, Kamm RD (2012) Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol (Camb) 4(8):863–874. doi:10.1039/c2ib00184e
Helm CLE, Fleury ME, Zisch AH, Boschetti F, Swartz MA (2005) Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA 102(44):15779–15784. doi:10.1073/pnas.0503681102
Katsumi A, Orr AW, Tzima E, Schwartz MA (2004) Integrins in mechanotransduction. J Biol Chem 279(13):12001–12004. doi:10.1074/jbc.R300038200
Shamloo A, Xu H, Heilshorn S (2012) Mechanisms of vascular endothelial growth factor-induced pathfinding by endothelial sprouts in biomaterials. Tissue Eng Part A 18(3–4):320–330. doi:10.1089/ten.TEA.2011.0323
Acknowledgements
This work was supported by grants from the National Institutes of Health (UH3 TR00048, R01 CA170879, and F31 CA163049). We would like to thank Mr Vinson Tran, Mo Kebaili, Linda McCarthy (University of California, Irvine) and Sandra Lam (Washington University in St. Louis) for technical assistance. We would also like to thank Dr. Abraham Lee for helpful advice and material assistance during the development stages of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shirure, V.S., Lezia, A., Tao, A. et al. Low levels of physiological interstitial flow eliminate morphogen gradients and guide angiogenesis. Angiogenesis 20, 493–504 (2017). https://doi.org/10.1007/s10456-017-9559-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9559-4