Abstract
In our study we assessed the tick burden on roe deer (Capreolus capreolus L.) in relation to age, physical condition, sex, deer density and season. The main objective was to find predictive parameters for tick burden. In September 2007, May, July, and September 2008, and in May and July 2009 we collected ticks on 142 culled roe deer from nine forest departments in Southern Hesse, Germany. To correlate tick burden and deer density we estimated deer density using line transect sampling that accounts for different detectability in March 2008 and 2009, respectively. We collected more than 8,600 ticks from roe deer heads and necks, 92.6% of which were Ixodes spp., 7.4% Dermacentor spp. Among Ixodes, 3.3% were larvae, 50.5% nymphs, 34.8% females and 11.4% males, with significant seasonal deviation. Total tick infestation was high, with considerable individual variation (from 0 to 270 ticks/deer). Adult tick burden was positively correlated with roe deer body indices (body mass, age, hind foot length). Significantly more nymphs were found on deer from forest departments with high roe deer density indices, indicating a positive correlation with deer abundance. Overall, tick burden was highly variable. Seasonality and large scale spatial characteristics appeared to be the most important factors affecting tick burden on roe deer.
Similar content being viewed by others
Introduction
European roe deer (Capreolus capreolus L.) are very common all over Europe. Their distribution ranges from southern Spain to northern Scandinavia, to the Ural mountains in Russia and to scattered populations in Turkey, Israel and Jordan (Linnell et al. 1998). As generalist herbivores, roe deer are able to feed on a wide variety of plants and thus live in several kinds of habitats. Many of these habitats are also occupied by certain tick species (mostly Ixodes spp. (Latreille, 1795) and Dermacentor spp. (Koch, 1844)) and roe deer are important hosts for ticks (Jensen et al. 2000; Walker et al. 2001; Rizzoli et al. 2007, Zeman and Pecha 2008). The ticks may profit from roe deer social behaviour and diurnal activities. Adult roe deer are territorial in spring and summer, offering a constant and reliable blood supply for tick development. High density roe deer populations are very common in Central Europe and are becoming more and more common in other parts of Europe (Andersen et al. 1998). Territorial behaviour includes chasing away subadult or subdominant individuals, providing the chance for ticks to be distributed quickly and effectively over long distances. Roe deer are known to migrate more than 100 km (Linnell et al. 1998), distances up to a few kilometres are usual for large parts of continental Europe. The preference of roe deer for dense vegetation and their diurnal rhythm of feeding and resting phases make them to easily accessible hosts for questing ticks. Roe deer have also learned to deal with and in many cases to profit from humans and their activities. It is one of the most important hunting game in Europe. Roe deer, along with other mammals (i.e. small rodens and dogs, Silaghi et al. 2008), are therefore important vectors for ticks and human tick borne diseases (TBDs). At a large spatial scale, a positive relationship between Lyme disease incidence in humans and roe deer density has been shown (Linard et al. 2007). Tick borne encephalitis (TBE) incidence in humans is also statistically associated with roe deer density (Rizzoli et al. 2009). Data on the role of deer on the transmission of TBDs are equivocal, however. For some of the tick-borne pathogens (for a review see Jongejan and Uilenberg 2004) roe deer are competent hosts, and for others roe deer might provide a cofeeding platform (Randolph et al. 1996; Kimura et al. 1995; Bruno et al. 2000). Although there is evidence for a correlation between deer and tick densities (Carpi et al. 2008), there is surprisingly little information on the tick burden of roe deer in general and its variation according to individual deer characteristics. Knowing the links might contribute to understand and to quantify the risk of tick borne diseases. It is also unknown how many ticks can be supported by roe deer. In general there is a lack of data on the effects of tick-borne diseases on roe deer. Some researchers state that roe deer are not susceptible to TBDs like tick borne encephalitis (TBE, Labuda et al. 2002; Hartemink et al. 2008; Rizzoli et al. 2009) or Lyme disease (Hartemink et al. 2008; Pugliese and Rosà 2008). Malandrin et al. (in press) could recently identify Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer blood and could clearly separate this Babesia species from others. Babesia capreoli can be fatal for roe deer, but does not pose a threat to either humans or livestock.
In this paper we aimed at testing the following hypotheses for each development stage/ sex of Ixodes and Dermacentor ticks and for the combined Ixodes and Dermacentor tick burden:
-
1.
tick burden underlies seasonal variation
-
2.
tick burden is influenced by individual characteristics of roe deer (sex, age, body mass, hind foot length)
-
3.
tick burden reflects roe deer density
Materials and methods
Study area
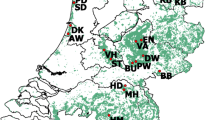
Ticks from roe deer were collected in nine different forest departments located in three different forest districts in South Hesse, Germany (Fig. 1). Site characteristics are summarised in Table 1. The forests are located within high risk areas for TBE (Robert Koch-Institute 2007). Mean size of the study sites is 1150 ha (range: 520–1,710 ha). In all nine forest departments, roe deer are abundant. Annual hunting bags ranged in the hunting season 2007/2008 between 23 and 60 roe deer per forest department (3.5–6.7/100 ha). Roe deer density indices computed after distance sampling in early spring at each of the study sites (see “Estimating roe deer density indices”) ranged from 2.4–9.1 roe deer /100 ha. Even within forest districts roe deer densities are quite variable, reflecting different habitat types, hunting regimes and possibly interspecific competition with other ungulate species. In three forest departments in the forest district Beerfelden, red deer (Cervus elaphus L.) with a hunting bag of 1.4–2.8 heads/100 ha in 2007/2008 is also common.
Tick sampling and assessment of roe deer data
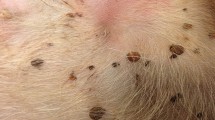
In September 2007 (n = 23), May 2008 (n = 47), July 2008 (n = 9), September 2008 (n = 23), May 2009 (n = 37) and July 2009 (n = 3) we sampled during 10 days each period ticks from 142 (= n total) hunter killed roe deer. Deer were stored in six different central cold storages of the forest districts which were maintained by the research team once a day. For 20 deer, the heads or the carcasses were removed by the hunters prior to investigation. They could only partly be screened (either head or neck). Two observers intensively investigated 138 necks and 126 heads from a total of 142 roe deer for tick infestation for a maximum time of 30 min each (Fig. 2). A preliminary study showed that roe deer from deciduous and coniferous forests in the region were mostly infested with ticks on their heads and necks. Ticks were removed with tweezers and tick-hooks and were collected in sterile tubes, separated by host individual, tick development stage and sex of adult ticks, and then stored at −80°C. As we collected thousands of ticks and some tick species are very similar, we separated them in the field at the genus level between Ixodes spp. and Dermacentor spp. ticks. Keys for identification in the lab were used to confirm the field separation. All randomly chosen and identified ticks were either Ixodes ricinus (L.) or Dermacentor reticulatus (Fabricius).
We estimated the age of roe deer by tooth wear (Mysterud and Østbye 2006), weighed them, and measured their hind foot length (HFL, Zannèsse et al. 2006). Furthermore, we assessed obvious health problems or physical damage through visual examination of the whole carcasses and by remarks from the hunters.
Estimating roe deer density indices
We estimated relative densities of roe deer using line transect methodology (Buckland et al. 2001) and subsequent analyses with the software package Distance 5 Release 2 (Thomas et al. 2006). In early March 2008 and 2009, we drove a fixed circuit in each forest area (mean ± SD: 18.3 ± 3.3 km); each circuit was driven twice on consecutive nights. We conducted each count with three persons: one person driving slowly (~6–12 km h−1) and observing animals on the transect line and two persons sitting on the top of the vehicle scanning both sides of the transect line with handheld spotlights (12 V, 55 W). In order to model detection functions, we estimated the perpendicular distance between the initial position of the deer and the transect using the cosine function (Buckland et al. 2001). We measured sighting distances with a laser rangefinder and sighting angles with a compass.
Acknowledging that this approach violates some of the distance sampling assumptions [i.e. transects are not distributed randomly, perfect detection on the line not given due to evasive behaviour of roe deer (Ward et al. 2004) or due to avoidance of roads by roe deer], we consider our estimates not as absolute density but as indices which allow comparisons of roe deer densities among different forest areas and years. Because the number of roe deer sightings/forest area/year was low (mean: 15.8 ± 6.4 SD), we pooled roe deer sightings according to the predominant terrain (‘hilly’ vs. ‘flat’) of the forest area. Based on AIC-values, these pooled detection functions performed better than forest area specific detection functions. We discarded the largest 5% of the distances and used half-normal key function with cosine series expansion to fit the detection functions. Using these stratum-specific detection functions and the size-bias regression method to estimate cluster size, we estimated area and year specific roe deer densities.
Predictive models for tick burden on roe deer
In order to explain the variation of ticks, we applied generalised linear models (GLM, Dobson and Barnett 2008) in SPSS (Version 17.0). Each model was fitted using a negative binomial error distribution (Shaw et al. 1998; Carpi et al. 2008) of the response variables ‘total Ixodes larvae’, ‘total Ixodes nymphs’, ‘total Ixodes females’, ‘total Ixodes males’, ‘total Ixodes’ and ‘total Dermacentor’. We did not subdivide Dermacentor data due to rare occurrence. We tested effects of the study sites (forest department), sampling month, roe deer density, sex, age (in months), hind foot length (cm) and disembowelled body mass (kg). Univariate relationships between two variables were further tested by Kendall’s Tau. Differences between variable values were tested by using the Mann–Whitney U-test.
Results
Overall tick burden
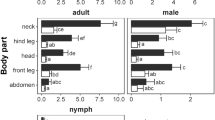
In total we collected 8,611 ticks from roe deer. Tick numbers ranged from 0 to 270 ticks per deer (head and neck only, Table 2), with an average of 65 ticks. 92.6% belonged to the Ixodes genus, 7.4% to the Dermacentor genus. We found all tick stages (Fig. 3a, b). Most of the ticks were nymphs (50.5%), followed by females (34.8%) males (11.4%), and larvae (3.3%). Most of the attached (feeding or questing) ticks were found on the roe deer’s heads (61%).
Individual variation
Adult tick burden was positively correlated with roe deer body indices such as body mass, age and hind foot length, with significantly more adult ticks on older and heavier animals with higher hind foot length (Table 3). Overall, investigated male roe deer carried more ticks than female roe deer. This result was, however, biased by the different hunting seasons of male (from May to October) and female roe deer (in May, only yearlings, and from September to January). In September, when both males and females were hunted, there were no significant differences in tick burden (38 vs. 31 ticks/deer, on average; P < 0.05, Mann–Whitney U-Test).
Spatial and seasonal variation
The variation between total tick infestations was not significant on the forest district level with 59–85 ticks/deer, on average. However, total larvae burden on roe deer was significantly (P < 0.05, Kendall’s Tau) higher in Dieburg than in Beerfelden and Lampertheim (Table 3). Although the factor ‘Forest Department’ had no direct effect on combined tick burden, significantly more nymphs were found on deer from forest departments with high roe deer density indices (RDI, Table 3), reflecting a positive correlation with deer abundance.
Roe deer were highly infested with ticks in May, whereas tick burden was lowest in September (Table 4). This was obvious for the total tick burden and the Ixodes genus. However, Dermacentor infestation was highest in July.
In May and September, most of the Ixodes ticks were nymphs, whereas in July the number of female Ixodes was slightly higher (Fig. 4). The number of male Ixodes was consistently about 1/3 of the number of females. Ixodes larvae burden peaked in July, as well as for all Dermacentor stages.
Predictive modelling
Computing single factor correlations (see “Spatial and seasonal variation”) does not account for ecological interaction of factors and is thus not helpful for general prediction of tick burden on roe deer. We therefore use generalised linear models (GLM) with the response variables ‘Ixodes larvae’, ‘Ixodes nymphs’, ‘Ixodes males’, ‘Ixodes females’, ‘Ixodes total’, ‘Dermacentor total’ and ‘ticks total’, the categorical variables ‘forest district’, ‘month’, ‘roe deer sex’ and the covariates ‘age’, ‘hind foot length (HFL)’, ‘body mass’ and ‘roe deer density index (RDI)’(Table 5).
Larval Ixodes tick burden varied significantly on the forest district level and by season. Roe deer age was negatively correlated with larval burden. Ixodes nymph burden showed a significant seasonality and was positively correlated with roe deer density indices. Adult Ixodes burden was positively correlated with roe deer body mass; female burden showed significant seasonality, however, male burden did not. Overall, Ixodes burden was only significantly affected by season. Total Dermacentor burden showed significant variation at the spatial (Forest District) and temporal (month) scale, and was negatively correlated with roe deer density indices and roe deer age.
The variables ‘sex’ and ‘roe deer hind foot length’ had no significant effect on tick burden.
Discussion
The general tick burden of roe deer in the study area was high. Overall, tick burden on roe deer appears to be highly variable with seasonality being the major factor explaining the variation of tick burden. Considering that we only sampled the deer’s heads and necks, the reported tick abundance per roe deer reflects ca. 60% of the total burden. In another study we found the deer’s head and neck to account for 47.28 % (SE ± 3.55) and 13.29% (±1.74) of the total Ixodes burden respectively (Kiffner et al. in press).
Modelling tick burden mainly revealed seasonal and spatial variation. It appears that individual host characteristics do not have the expected high effects on tick burden. However, Ixodes larvae and total Dermacentor numbers were negatively correlated with deer age, indicating a preference for younger hosts. We hypothesize that this is caused by behavioural differences (younger deer have longer resting phases, especially as fawns) and by the thinner skin of younger animals. Adult tick burden is positively correlated with the roe deer body mass, being in accordance with the results of the parasite-host metaanalysis done by Poulin and George-Nascimento (2007). As the male tick burden is mostly triggered by the female burden, effects on both sexes are almost the same. The negative influence of the roe deer density index on total Dermacentor burden might be explained by spatial differences in Dermacentor distribution, coincidently overlapping with also highly significant forest district effects. The sex of roe deer does not have any significant effect on tick parasitism. Schalk and Forbes (1997) generally found small differences in parasitism between the sexes of mammals. Schmidtmann et al. (1998) reported male biased tick parasitism (Ixodes scapularis) on white-tailed deer (Odocoileus virginianus). This deer species, however has a more pronounced sexual size dimorphism than roe deer (Geist 1998) and thus male-biased parasitism might actually be an artefact of sexual size dimorphism.
We did not observe any specific sign of deer health problems caused by high tick infestation. At least for Lyme borreliosis and TBE, roe deer are not competent hosts (Labuda et al. 2002; Hartemink et al. 2008; Pugliese and Rosà 2008; Rizzoli et al. 2009). However, roe deer are competent reservoirs for Anaplasma phagocytophilum causing a febrile disease (Silaghi et al. 2008), and for Babesia capreoli (Malandrin et al. in press).
Carpi et al. (2008) screened parts of the forelegs of roe deer from Northeastern Italy for tick infestation. They found very high tick numbers as well (up to 388 Ixodes ricinus/deer), although almost 90% were larvae. As we could also find all life stages of ticks (larvae, nymphs and adults) feeding on the same individual and sometimes aggregated very closely together (<1 cm), the chance of co-feeding (Randolph 2004) and TBE virus transmission from infected nymphs to larvae or even females to nymphs and larvae should be further considered. In spite of roe deer being a non-competent host for TBE, it is already being used as an ideal and easily available sentinel animal for TBE distribution using serological investigation (Gerth et al. 1995; Labuda et al. 2002; Carpi et al. 2008).
Walker et al. (2001) removed ticks from roe deer forelegs. Similar to our study, months with the highest larvae infestation were July and August (mean ~58 larvae per roe deer leg) and most nymphs were collected in May (mean ~16 nymphs per leg). The seasonal variation of tick densities in general and of tick development stages questing or feeding on roe deer followed the marked seasonality in tick population dynamics including diapauses and in environmental conditions in temperate zones (MacLeod 1939; Lees and Milne 1951; Gray 1971; Kalsbeek and Frandsen 1996). In our study Ixodes nymph, female and male numbers on roe deer peaked in May, whereas most larvae were counted in July. This corresponds well with data presented by Randolph (2004) for Ixodes ricinus, although she could show a very high interannual variability as well.
As far as we know, only one further publication reports on roe deer infestation with Dermacentor ticks (Dautel et al. 2006). In their study D. reticulatus was found on 23 deer out of 721 deer from all over Germany. Most of the infested animals were red deer (Cervus elaphus) and only a few roe deer. Their results showed that D. reticulatus is much more common in Germany than previously known. In Hungary this tick species is also expanding its geographic range, and D. reticulatus-borne diseases (e.g. tularemia and tick-borne lymphadenopathy) are a concern in the region (Sréter et al. 2005). Dermacentor reticulatus is better known as a vector for the pathogens Babesia canis and Ehrlichia canis, causing diseases mainly in dogs (Ogden et al. 2000). They can also be vector for Coxiella burnetii causing Q fever in domestic animals and even in humans (Movila et al. 2006), and for Rickettsia (Dautel et al. 2006). Borrelia have been found in D. reticulatus (Kahl et al. 1992), but this tick species is apparently not an effective vector for Borrelia (Jongejan and Uilenberg 2004). In our study roe deer were most infested with Dermacentor ticks in July. The high risk potential for several diseases and their activity in mid-summer when people frequently enter the forests for recreation make further studies on the ecology of this tick species and the role of wildlife for its population dynamics indispensable.
The question whether roe deer abundance enhances tick abundance and the subsequent risk for tick borne diseases can not be satisfactorily answered. The fact that we found a significant positive correlation between Ixodes nymph numbers on roe deer and roe deer density indices does not prove to be an obligate relationship, since other factors (e.g. small rodent densities as more important hosts for larvae, site conditions or habitat structures) could be more important. The same factors could also explain the increasing TBE risk in recent years which correlate with increasing roe deer densities in the Italian Alps (Rizzoli et al. 2009), as both could have been made possible by other factors and the following distribution of both ticks and roe deer in higher altitudes. Carpi et al. (2008) concluded that tick infestation of roe deer is very site specific, but not necessarily dependent on roe deer densities. This might even explain the negative correlation of roe deer density indices and Dermacentor numbers on roe deer in our study. An exclusively positive influence of roe deer densities on ticks has rarely been shown. Jensen et al. (2000) found a positive correlation of Ixodes nymph density and roe deer abundance. Ostfeld et al. (2006) could only confirm a weak effect of white tailed deer (Odocoileus virginianus) abundance on Ixodes scapularis larvae and state that there is a clear decoupling of stage specific abundances. Walker et al. (2001) even found negative correlations between densities of roe deer and nymphs. Gray et al. (1992) showed that the availability of deer as hosts has a major impact on tick densities, but even small numbers of deer can maintain very large tick populations (Wilson et al. 1984; Robertson et al. 2000). A white-tailed deer reduction by almost 50% had no apparent effect on questing Ixodes scapularis numbers (Jordan et al. 2007).
All roe deer of our study were culled in forested areas. However, home ranges of roe deer are relatively large (10—more than 200 ha (Lovari and San José 1997; Mysterud 1999)) and usually cover more than one habitat type under Central European conditions, thus impeding the identification of habitat specific effects on roe deer tick burden with our dataset.
To better predict tick densities we suggest including more specific habitat characteristics such as soil moisture and grinding vegetation cover.
References
Andersen R, Duncan P, Linnell JDC (1998) The European roe deer: the biology of success. Scandinavian University Press, Oslo
Bruno P, Bruno G, Peréz-Eid C (2000) Detection of spirochaetes of Borrelia burgdorferi complexe in the skin of cervids by PCR and culture. Eur J Epidemiol 16:869–873
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2001) Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford, UK
Carpi G, Cagnacci F, Neteler M, Rizzoli A (2008) Tick infestation on roe deer in relation to geographic and remotely sensed climatic variables in a tick-borne encephalitis endemic area. Epidemiol Infect 136:1416–1424
Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E (2006) Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int J Med Microbiol 296(Suppl. 1):149–156
Dobson AJ, Barnett AG (2008) An introduction to generalized linear models, 3rd edn. Chapman & Hall/CRC, Boca Raton
Geist V (1998) Deer of the world: their evolution, behavior, and ecology, stackpole books. Mechanicsbarg, USA, p 421
Gerth HJ, Grimshandl D, Stage B, Döller G, Kunz C (1995) Roe deer as sentinels for endemicity of tick-borne encephalitis virus. Epidemiol Infect 115:355–365
Gray JS (1971) The development and seasonal activity of the tick Ixodes ricinus: a vector of Lyme Borreliosis. Rev Med Vet Ent 79:323–333
Gray JS, Kahl O, Janetzki C, Stein J (1992) Studies on the ecology of Lyme disease in a deer forest in County Galway, Ireland. J Med Entomol 29:915–920
Hartemink NA, Randolph SE, Davis SA, Heesterbeek JAP (2008) The basic reproduction number for complex disease systems: Defining R0 for tick-borne infections. Am Nat 171:743–754
Jensen PM, Hansen H, Frandsen F (2000) Spatial risk assessment for Lyme borreliosis in Denmark. Scan J Infect Dis 32:545–550
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14
Jordan RA, Schulze TL, Jahn MB (2007) Effects of reduced deer density on the abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease incidence in a northern New Jersey endemic area. J Med Entomol 44:752–757
Kahl O, Janetzki C, Gray JS, Stein J, Bauch RJ (1992) Tick infection rates with Borrelia: Ixodes ricinus versus Haemaphysalis concinna and Dermacentor reticulates in two locations in eastern Germany. Med Vet Entomol 6:363–366
Kalsbeek V, Frandsen F (1996) The seasonal activity of Ixodes ricinus ticks in Denmark. Anzeiger für Schädlingskunde (J Pest Sci) 69:160–161
Kiffner C, Lödige C, Alings M, et al (in press) Abundance estimation of Ixodes ticks (Acari: Ixodidae) on roe deer (Capreolus capreolus). Exp Appl Acarol
Kimura K, Isogai E, Isogai H et al (1995) Detection of Lyme disease spirochetes in the skin of naturally infected wild sika deer (Cervus nippon yesoensis). Appl Environ Microbiol 61:1641–1642
Koch-Institute Robert (2007) FSME: risikogebiete in Deutschland. Epidemiol Bull 15:129–135
Labuda M, Elecková E, Licková M, Sabó A (2002) Tick-borne encephalitis virus foci in Slovakia. Int J Med Microbiol 291:43–47
Lees AD, Milne A (1951) The seasonal and diurnal activities of individual sheep ticks (Ixodes ricinus L.). Parasitology 41:189–208
Linard C, Lamarque P, Heyman P, Ducoffre G, Luyasu V et al (2007) Determinants of the geographic distribution of Puumula virus and Lyme borreliosis infections in Belgium. Int J Health Geo 6:15. doi:10.1186/1476-072x-6-15
Linnell JDC, Wahlström K, Gaillard JM (1998) From birth to independence: birth, growth, neonatal mortality, hiding behaviour and dispersal. In: Andersen R et al (eds) The European roe deer: the biology of success. Scandinavian University Press, Oslo, pp 257–284
Lovari S, San José C (1997) Wood dispersion affects home range size of female roe deer. Behav Proc 40:239–241
MacLeod J (1939) The seasonal and annual incidence of the sheep Tick, Ixodes ricinus, in Britain. Bull Entomol Res 30:103–118
Malandrin L, Jouglin M, Sun Y, Brisseau N, Chauvin A (in press) Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. doi:10.1016/j.ijpara.2009.08.008
Movila A, Uspenskaia I, Toderas I, Melnic V, Conovalov J (2006) Prevalence of Borrelia burgdorferi sensu lato and Coxiella burnetii in ticks collected in different biocenoses in the Republic of Moldova. Int J Med Microbiol 296(Suppl. 1):172–176
Mysterud A (1999) Seasonal migration pattern and home range of roe deer (Capreolus capreolus) in an altitudinal gradient in southern Norway. J Zool 247:479–486
Mysterud A, Østbye E (2006) Comparing simple methods for ageing roe deer Capreolus capreolus: are any of them useful for management? Wildl Biol 12:101–107
Ogden NH, Cripps P, Davison CC, Owen G, Parry JM, Timms BJ, Forbes AB (2000) The ixodid tick species attaching to domestic dogs and cats in Great Britain and Ireland. Med Vet Entomol 14:332–338
Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F (2006) Climate, deer, rodents and acorns as determinants of variation in lyme-disease risk. PloS Biol 4:1058–1068
Poulin R, George-Nascimento M (2007) The scaling of total parasite biomass with host body mass. Int J Parasitol 37:359–364
Pugliese A, Rosà R (2008) Effect of host populations on the intensity of ticks and the prevalence of tick-borne pathogens: how to interpret the results of deer exclosure experiments. Parasitology 135:1531–1544
Randolph SE (2004) Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129:37–65
Randolph SE, Gern L, Nuttall PA (1996) Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parastitol Today 12:472–479
Rizzoli A, Neteler M, Rosà R, Versini W, Cristofolini A, Bregoli M, Buckley A, Gould EA (2007) Early detection of tick-borne encephalitis virus spatial distribution and activity in the province of Trento, northern Italy. Geospatial Health 2:169–176
Rizzoli A, Hauffe HC, Tagliapietra V, Neteler M, Rosà R (2009) Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLoS ONE 4:e4336. doi:10.1371/journal.pone.0004336
Robertson JN, Gray JS, Stewart P (2000) Tick bite and Lyme borreliosis risk at a recreational site in England. Eur J Epidemiol 16:647–652
Schalk G, Forbes MR (1997) Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos 78:67–74
Shaw DJ, Grenfell BT, Dobson AP (1998) Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597–610
Silaghi C, Gilles J, Höhle M, Fingerle V, Just FT, Pfister K (2008) Anaplasma phagocytophilum infection in Ixodes ricinus, Bavaria, Germany. Em Inf Dis 14:972–974
Sréter T, Széll Z, Varga I (2005) Spatial distribution of Dermacentor reticulatus and Ixodes ricinus in Hungary: evidence for change? Vet Parasitol 128:347–351
Thomas L, Laake JL, Strindberg S, Marques FFC, Buckland ST, Borchers DL, Anderson DR, Burnham KP, Hedley SL, Pollard JH, Bishop JRB, Marques TA (2006) Distance 5.0. Release 2. Research Unit for Wildlife Population Assessment, University of St. Andrews, UK. http://www.ruwpa.st-and.ac.uk/distance/
Walker AR, Alberdi MP, Urquhart KH, Rose H (2001) Risk factors in habitats of the tick Ixodes ricinus influencing human exposure to Ehrlichia phagocytophila bacteria. Med Vet Entomol 15:40–49
Ward AI, White PCL, Critchley CH (2004) Roe deer Capreolus capreolus behaviour affects density estimates from distance sampling surveys. Mam Rev 34:315–319
Wilson ML, Levine JF, Spielman A (1984) Effect of deer reduction on abundance of the deer tick (Ixodes dammini). Yale J Biol Med 57:697–705
Zannèsse A, Baïsse A, Gaillard J-M, Hewison AJM, Saint-Hillaire K, Toïgo C, van Laere G, Morellet N (2006) Hind foot length: an indicator for monitoring roe deer populations at a landscape scale. Wildl Biol Bull 34:351–358
Zeman P, Pecha M (2008) Segregation of genetic variants of Anaplasma phagocytophilum circulating among wild ruminants within a Bohemian forest (Czech Republic). Int J Med Microbiol 298(Suppl. 44):203–210
Acknowledgments
This study is funded by the German Federal Ministry of Education and Research (BMBF) within the “Research on Zoonotic Infectious Diseases” programme, “Emerging arthropod-borne-viral infections in Germany: Pathogenesis, diagnostics and surveillance” (grant no. 1363120) and greatly supported by the State Forest Administration of Hesse. We are also grateful to S. Bauling, K. Ehlmann, M. Ksinsik, A., C. and L. Lödige, A.-L. Schäfer, E. Rühe and M. Scholz for their help during field work, and Kenneth Elgersma for editing the English.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Vor, T., Kiffner, C., Hagedorn, P. et al. Tick burden on European roe deer (Capreolus capreolus). Exp Appl Acarol 51, 405–417 (2010). https://doi.org/10.1007/s10493-010-9337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-010-9337-0