Abstract
Background. Imatinib mesylate is a potent inhibitor of Abl, KIT, and PDGFR tyrosine kinases. Breast cancer has variable expression of KIT and PDGFR therefore we conducted a phase II trial to evaluate the safety and efficacy of imatinib in patients with metastatic breast cancer (MBC).
Patients and methods. Eligible patients had measurable and progressive MBC, with no limits on prior chemo- or hormonal therapy. Imatinib was initially administered at a dose of 400 mg orally twice a day with provisions for dose reductions based on toxicities. The primary endpoint was clinical benefit based on RECIST criteria. Tumor specimens were tested for expression of KIT and PDGFR tyrosine kinases.
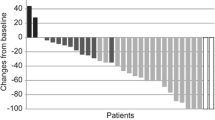
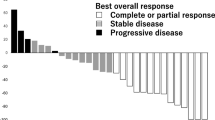
Results. Sixteen patients were enrolled and treated. Median age was 55 years (range: 35–73); median number of prior chemotherapy regimens for MBC was 4 (range 1–8). The main non-hematologic toxicities were (Grades 1/2; Grade 3): fatigue (56%; 6%), edema (38%; 19%), nausea (31%; 19%), vomiting (38%; 0%), anorexia (38%; 0%), diarrhea (19%; 6%), and rash (25%; 6%). Grade 3/4 hematologic and biochemical abnormalities were minimal. There was no evidence of clinical benefit. The median duration of therapy on trial was 28 days (range 2–71). Of the 13 testable cases: 1 was KIT positive and 4 were PDGFR positive.
Conclusion. Imatinib therapy at doses of 800 mg/day was associated with significant toxicity in patients with heavily pre-treated MBC. Our results do not indicate activity for imatinib monotherapy in these unselected patients.
Similar content being viewed by others
References
J Schlessinger (2000) ArticleTitleCell signaling by receptor tyrosine kinases Cell 103 211–225 Occurrence Handle10.1016/S0092-8674(00)00114-8 Occurrence Handle1:CAS:528:DC%2BD3cXns1Cltrs%3D Occurrence Handle11057895
Y Yarden MX Sliwkowski (2001) ArticleTitleUntangling the ErbB signalling network Nat Rev Mol Cell Biol 2 IssueID2 127–137 Occurrence Handle10.1038/35052073 Occurrence Handle1:CAS:528:DC%2BD3MXivVWnt7k%3D Occurrence Handle11252954
M Mauro M O’Dwyer MC Heinrich BJ Druker (2002) ArticleTitleSTI571: a paradigm of new agents for cancer therapeutics J Clin Oncol 20 IssueID1 325–334
MC Heinrich DJ Griffith BJ Druker et al. (2000) ArticleTitleInhibition of c-kit receptor tyrosine kinase activity by STI571, a selective tyrosine kinase inhibitor Blood 96 925–932 Occurrence Handle1:CAS:528:DC%2BD3cXltlKitLw%3D Occurrence Handle10910906
TF Franke SI Yang TO Chan et al. (1995) ArticleTitleThe protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase Cell 81 IssueID5 727–736 Occurrence Handle1:CAS:528:DyaK2MXmtFOiur8%3D Occurrence Handle7774014
T Jahn P Seipel S Urschel et al. (2002) ArticleTitleRole for the adaptor protein Grb10 in the activation of Akt Mol Cell Biol 22 IssueID4 979–991
F Gesbert WR Sellars S Signoretti et al. (2000) ArticleTitleBCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-kinase/AKT pathway J Biol Chem 275 IssueID50 39223–39230
BJ Druker M Talpaz D Resta et al. (2001) ArticleTitleEfficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia N Engl J Med 344 IssueID14 1031–1037 Occurrence Handle10.1056/NEJM200104053441401 Occurrence Handle1:CAS:528:DC%2BD3MXivFGnu70%3D Occurrence Handle11287972
BJ Druker CL Sawyer H Kantarjian et al. (2001) ArticleTitleActivity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the philadelphia chromosome N Engl J Med 344 IssueID14 1038–1042 Occurrence Handle10.1056/NEJM200104053441402 Occurrence Handle1:CAS:528:DC%2BD3MXivFGnu7o%3D Occurrence Handle11287973
H Joensuu S Dimitrijevic (2001) ArticleTitleTyrosine kinase inhibitor imatinib (STI571) as an anticancer agent for solid tumors Ann Med 33 451–455
J Lasota A Wozniak M Sarlomo-Rikala et al. (2000) ArticleTitleMutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors Am J Pathol 157 IssueID4 1091–1095 Occurrence Handle1:CAS:528:DC%2BD3cXnsVKjtbg%3D Occurrence Handle11021812
H Joensuu PJ Roberts M Sarlomo-Rikala et al. (2001) ArticleTitleEffect of the tyrosine kinase inhbitor STI571 in a patient with a metastatic gastrointestinal stromal tumor N Engl J Med 344 IssueID14 1052–1056 Occurrence Handle10.1056/NEJM200104053441404 Occurrence Handle1:STN:280:DC%2BD3M3gvVekuw%3D%3D Occurrence Handle11287975
A Oosterom ParticleVan I Judson J Verweij et al. (2001) ArticleTitleSafety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumors: a phase I study Lancet 358 1421–1423 Occurrence Handle10.1016/S0140-6736(01)06535-7 Occurrence Handle11705489
CD Blanke M Mehren ParticleVon H Joensuu et al. (2001) ArticleTitleEvaluation of the safety and efficacy of an oral molecularly-targeted therapy, STI571, in patients with unresectable or metastatic astrointestinal stromal tumors (GISTs) expressing c-KIT (CD117) Proc Am Soc Clin Oncol 20 1a
SJ Hines C Organ MJ Kornstein GW Krystal (1995) ArticleTitleCoexpression of the c-Kit and stem cell factor genes in breast carcinomas Cell Growth Diff 6 769–779
S Palmu KO Soderstrom K Quazi et al. (2002) ArticleTitleExpression of C-KIT and HER-2 tyrosine kinase receptors in poor–prognosis breast cancer Anticancer Res 22 IssueID1A 411–414
B Bhardwaj J Klassen N Cossette (1996) ArticleTitleLocalization of platelet-derived growth factor β receptor expression in the periepithelial stroma of human breast carcinoma Clin Can Res 2 773–782
AT Oosterom Particlevan IR Judson J Verweij et al. (2002) ArticleTitleUpdate of phase I study of imatinib (STI571) in advanced soft tissue sarcoma and gastrointestinal stromal tumors: a report of the EORTC soft Tissue and Bone Sarcoma Group. Eur J Cancer 38 (Suppl 5) S83–S87
B Peng M Hayes A Racine-Poon et al. (2001) ArticleTitleClinical investigation of the pharmacokinetic/pharmacodynamic relationship for Glivec (STI571): a novel inhibitor for signal transduction Proc Am Soc Clin Oncol 20 71a
P Therasse SG Arbuck EA Eisenhauer et al. (2000) ArticleTitleNew guidelines to evaluate the response to treatment in solid tumors J Nat Cancer Inst 92 IssueID3 205–216
PY Wen WK Yung K Hess et al. (2002) ArticleTitlePhase I study of STI571 (Gleevec) for patients with recurrent malignant gliomas and meningiomas (NABTC 00–08) Proc Am Soc Clin Oncol 21 73a
MC Heinrich C Corless CD Blanke et al. (2002) ArticleTitleKIT mutational status predicts clinical response to STI571 in patients with metastatic gastrointestinal stromal tumors (GISTs) Proc Am Soc Clin Oncol 21 2a
R Simon S Panussis R Maurer et al. (2004) ArticleTitleKIT (CD117)-positive breast cancers are infrequent and lack KIT gene mutations Clin Cancer Res 10 178–183
Author information
Authors and Affiliations
Corresponding author
Additional information
Previously published: Modi S, Seidman A, Dickler M, et al: A phase II trial of STI571 in patients with metastatic breast cancer (MBC). Proc Am Soc Clin Oncol 22: 2003 (Abstract 68); Previously presented: ASCO May 2003 (Poster Discussion); Research support: Novartis Pharmaceuticals Inc.
Rights and permissions
About this article
Cite this article
Modi, S., Seidman, A.D., Dickler, M. et al. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 90, 157–163 (2005). https://doi.org/10.1007/s10549-004-3974-0
Issue Date:
DOI: https://doi.org/10.1007/s10549-004-3974-0