Abstract
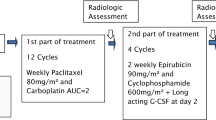
To explore the impact of dose intensity (DI) in the adjuvant setting of breast cancer, a randomized phase III trial was conducted comparing postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide, methotrexate and fluorouracil (CMF)in high-risk breast cancer patients. From Oct 2000 to June 2005, 1,121 node-positive patients were randomized to dose-dense sequential epirubicin 110 mg/m2 and paclitaxel (Taxol®, Bristol Myers-Squibb, Princeton, NJ) 250 mg/m2 (group A), or concurrent epirubicin 83 mg/m2 and paclitaxel 187 mg/m2 (group B), both followed by three cycles of “intensified” combination chemotherapy with CMF. By protocol design total cumulative dose and duration of treatment were identical in both groups. Dose intensity of epirubicin and paclitaxel was double in the dose-dense arm. Prophylactic treatment with granulocyte colony-stimulating factor was given with the dose-dense treatments. Disease-free survival (DFS) was the primary endpoint. At a median follow-up of 76 months, 253 patients (23%) had documented disease relapse (123 vs. 130 in groups A and B, respectively) and 208 deaths (101, group A and 107, group B) had been observed. The 5-year DFS rate of 74 and 74% and OS rate of 86 and 85% were observed for group A and group B, respectively. No differences were found in DFS or OS between the two treatment groups (P = 0.78 and P = 0.45 for DFS and OS, respectively). Safety analysis results showing that both regimens were well tolerated and safe have been previously published (Fountzilas et al. Ann Oncol 2008). No DFS or OS benefit from the dose-dense sequential epirubicin and paclitaxel was detected when compared to the concurrent administration of the same drugs. No additional safety issues were raised with long-term follow-up.




Similar content being viewed by others
References
Berry DA, Cronin KA, Plevritis SK et al (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792
Norton L (2001) Theoretical concepts and the emerging role of taxanes in adjuvant therapy. Oncologist 6(Suppl 3):30–35
Trudeaue M, Charbonneau F, Gelmon K et al (2005) Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol 6:886–898
Bonadonna G, Zambetti M, Valagussa P (1995) Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. JAMA 273:542–547
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Hudis C, Seidman A, Baselga J et al (1999) Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: feasibility and efficacy. J Clin Oncol 17:93–100
Founzilas G, Nicolaides C, Aravantinos G et al (1998) Dose-dense adjuvant chemotherapy with epirubicin monotherapy in patients with operable breast cancer and 10 positive axillary lymph nodes. A feasibility study. Oncology 55:508–512
Norton L, Simon R (1986) The Norton-Simon hypothesis revisited. Cancer Treat Res 70:163–169
Citron ML, Berry DA, Cirrincione C et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer; first report of Intergroup Trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 21:1431–1439
Fountzilas G, Dafni U, Gogas H et al (2007) Postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel and CMF in patients with high-risk breast cancer: safety analysis of the Hellenic Cooperative Oncology Group randomized Phase III trial HE 10/00. Ann Oncol 19:853–860
Coombes RC, Hall E, Gibson LJ et al (2004) Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Fountzilas G, Skarlos D, Dafni U et al (2005) Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 16:1762–1771
Moher D (1998) CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. JAMA 279:1489–1491
Hudis C, Fornier M, Riccio L et al (1999) 5-Year results of dose-intensive sequential adjuvant chemotherapy for women with high-risk node-positive breast cancer: a phase II study. J Clin Oncol 17:118–1126
Hryniuk W (2001) Dosage parameters in chemotherapy of breast cancer. Breast Dis 14:21–30
Piccart-Gebhart MJ (2003) Mathematics and Oncology: a match for life? J Clin Oncol 21:1425–1428
Trudeau ME (2001) Optimizing adjuvant breast cancer chemotherapy: rationale for the MA.21 study. Oncology (Williston Park) 5(Suppl. 7):7–13
Moebus V, Jackisch Ch, Luek HJ et al (2010) Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 28:2874–2880
Hudis C, Citron M, Berry D et al (2005) Five year follow-up of INT C9741: Dose dense chemotherapy is safe and effective. Breast Cancer Res Treat 94:S20 (suppl: abstr 41)
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Mamounas EP, Bryant J, Lembersky BC et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer. Results from NSABP-B28. J Clin Oncol 23:3686–3696
Fisher B, Anderson S, Wickerham DL et al (1996) Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer. Findings from national surgical adjuvant breast and bowel project B-22. J Clin Oncol 15:1858–1869
Fisher B, Anderson S, DeCillis A et al (1999) Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Finding from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol 17:3374–3388
Βonilla L, Ben-Aharon I, Vidal L et al (2010) Dose-Dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. JNCI 24:1845–1854
Venturini M, Del Mastro L, Aitini E et al (2005) Dose-dense adjuvant chemotherapy in early breast cancer patients. Results from a randomized trial. J Natl Cancer Inst 97:1724–1733
Levine MN, Pritchard KI, Bramwell VHC et al (2005) Randomized trial comparing cyclophosphamide, epirubicin and fluorouracil with cyclophosphamide, methotrexate and flouorouracil in premenopausal women with node-positive breast cancer. Update of National Cancer Institute of Canada Clinical Trial Group Trial MA5. J Clin Oncol 23:5166–5170
Bonneterre J, Roche H, Kerbrat P et al (2005) Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10 year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol 23:2686–2693
Linden HM, Haskell CM, Green SJ et al (2007) Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). J Clin Oncol 25(6):656–661
Burnell M, Levine MN, Chapman Jw et al (2010) Cyclophosphaimide, epirubicin and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol 28:77–82
Hayes DF, Thor AD, Dressler LG et al (2007) HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med 357:1496–1506
Berry DA, Cirrincione C, Henderson IC et al (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667
Gluz O, Nitz UA, Harbeck N et al (2008) Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol 19:861–870
Eiermann W, Pienkowski T, Crown J (2011) Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 29:3877–3884
Piedbois P, Serin D, Priou F et al (2007) Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol 18:52–57
Gluck S (2005) Adjuvant chemotherapy for early breast cancer: optimal use of epirubicin. Oncologist 10:780–791
Praga C, Bergh J, Biss J et al (2005) Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 23:4179–4191
Burstein HJ, Parker LM, Keshaviah A et al (2005) Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol 23:8340–8347
Smith TJ, Khatcheressian J, Lyman GH et al (2006) Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:1–19
Hershman D, Neugut Al, Jacobson JS et al (2007) Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 99:196–205
Chau D (2011) The End of an era: shall we move forward? JCO 3849–3851
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gogas, H., Dafni, U., Karina, M. et al. Postoperative dose-dense sequential versus concomitant administration of epirubicin and paclitaxel in patients with node-positive breast cancer: 5-year results of the Hellenic Cooperative Oncology Group HE 10/00 phase III Trial. Breast Cancer Res Treat 132, 609–619 (2012). https://doi.org/10.1007/s10549-011-1913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1913-4