Abstract
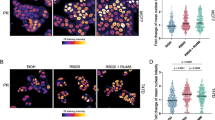
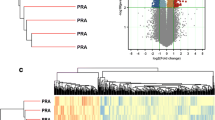
The receptor activator of nuclear factor-κB ligand (RANKL) acts as a paracrine factor in progesterone-induced mammary epithelial proliferation and tumorigenesis. This evidence comes mainly from mouse models. Our aim was to examine whether RANKL expression in human normal and malignant breast is under the control of progesterone throughout the menstrual cycle. Breast epithelial samples were obtained by random fine needle aspiration (rFNA) of the contralateral unaffected breasts (CUB) of 18 breast cancer patients, with simultaneous serum hormone measurements. Genes correlated with serum progesterone levels were identified through Illumina microarray analysis. Validation was performed using qRT-PCR in rFNA samples from CUB of an additional 53 women and using immunohistochemistry in tissue microarrays of 61 breast cancer samples. Expression of RANKL, DIO2, and MYBPC1 was correlated with serum progesterone in CUB, and was significantly higher in luteal phase. RANKL and MYBPC1 mRNA expression were highly correlated between CUB and matched tumor samples. RANKL protein expression was also significantly increased in the luteal phase and highly correlated with serum progesterone levels in cancer samples, especially in hormone receptor positive tumors. The regulatory effects of progesterone on the expression of RANKL, DIO2, and MYBPC1 were confirmed in three-dimensional cultures of normal breast organoids. In normal breast and in breast cancer, RANKL mRNA and protein expression fluctuate with serum progesterone with highest levels in the luteal phase, suggesting that RANKL is a modulator of progesterone signaling in normal and malignant breast tissue and a potential biomarker of progesterone action and blockade.





Similar content being viewed by others
References
Obr AE, Edwards DP (2012) The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 357:4–17
Schramek D, Sigl V, Penninger JM (2011) RANKL and RANK in sex hormone-induced breast cancer and breast cancer metastasis. Trends Endocrinol Metab 22:188–194
Fernandez-Valdivia R, Lydon JP (2012) From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol Cell Endocrinol 357:91–100
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Schneider P et al (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107:2989–2994
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R et al (2010) RANK ligand mediates progestin induced mammary epithelial proliferation and carcinogenesis. Nature 468:103–107
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ et al (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468:98–102
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL et al (2010) Progesterone induces adult mammary stem cell expansion. Nature 465:803–807
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER et al (2010) Control of mammary stem cell function by steroid hormone signaling. Nature 465:798–802
Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM et al (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470:548–553
Beral V (2003) Breast cancer and hormone-replacement therapy in the million women study. Lancet 362:419–427
Lee S, Kolonel L, Wilkens L, Wan P, Henderson B, Pike M (2006) Postmenopausal hormone therapy and breast cancer risk: the Multiethnic Cohort. Int J Cancer 118:1285–1291
Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH et al (2010) Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 304:1684–1692
Ferguson DJ, Anderson TJ (1981) Morphological evaluation of cell turnover in relation to the menstrual cycle in the ‘resting’ human breast. Br J Cancer 44:177–181
Ferguson DJ (1988) An ultrastructural study of mitosis and cytokinesis in normal ‘resting’ human breast. Cell Tissue Res 252:581–587
Anderson TJ, Battersby S, King RJ, McPherson K, Going JJ (1989) Oral contraceptive use influences resting breast proliferation. Hum Pathol 20:1139–1144
Anderson TJ, Ferguson DJ, Raab GM (1982) Cell turnover in the ‘resting’ human breast: influence of parity, contraceptive pill, age and laterality. Br J Cancer 46:376–382
Going JJ, Anderson TJ, Battersby S, MacIntyre CC (1988) Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol 130:193–204
Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M et al (1988) The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer 58:163–170
Bai M, Agnatis NJ, Kamina S, Demou A, Zagorianakou P, Katsaraki A et al (2001) In vivo cell kinetics in breast carcinogenesis. Breast Cancer Res 3:276–283
Feuerhake F, Sigg W, Höfter EA, Unterberger P, Welsch U (2003) Cell proliferation, apoptosis, and expression of Bcl-2 and Bax in non-lactating human breast epithelium in relation to the menstrual cycle and reproductive history. Breast Cancer Res Treat 77:37–48
Capuco AV, Ellis S, Wood DL, Akers RM, Garrett W (2002) Postnatal mammary ductal growth: three-dimensional imaging of cell proliferation, effects of estrogen treatment, and expression of steroid receptors in prepubertal calves. Tissue Cell 34:143–154
Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991
Russo J, Ao X, Grill C, Russo IH (1999) Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227
Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM (2000) C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol 14:359–368
Rajaram RD, Brisken C (2012) Paracrine signaling by progesterone. Mol Cell Endocrinol 357:80–90
Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI et al (2009) DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150:3318–3326
Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF et al (2013) Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med 5(182):182ra55
Ramakrishnan R, Khan SA, Badve S (2002) Morphological changes in breast tissue with menstrual cycle. Mod Pathol 15:1348–1356
Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ (1998) Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst 90:37–42
Lee O, Helenowski IB, Chatterton RT, Jovanovic B, Khan SA (2012) Prediction of menopausal status from estrogen-related gene expression in benign breast tissue. Breast Cancer Res Treat 131:1067–1076
Wang J, Gupta A, Hu H, Chatterton RT, Clevenger CV, Khan SA (2013) Comment on “Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med 5(215):215le4
Wang J, Scholtens D, Holko M, Ivancic D, Lee O, Hu H et al (2013) Lipid metabolism genes in contralateral unaffected breast and estrogen receptor status of breast cancer. Cancer Prev Res 6:321–330
Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Kamel S, Zeiger S et al (2000) Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst 92:1217–1227
Chatterton RT, Khan SA, Heinz R, Ivancic D, Lee O (2010) Patterns of sex steroid hormones in nipple aspirate fluid during the menstrual cycle and after menopause in relation to serum concentrations. Cancer Epidemiol Biomark Prev 19:275–279
Burger HG, Dudley EC, Robertson DM, Dennerstein L (2002) Hormonal changes in the menopause transition. Recent Prog Horm Res 57:257–275
Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006) Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med 44:883–887
Wood CE, Branstetter D, Jacob AP, Cline JM, Register TC, Rohrbach K et al (2013) Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res 15:R62
Brisken C (2013) Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer 13:385–396
Jankowitz RC, McGuire KP, Davidson NE (2013) Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer. Breast 22:S165–S170
Casula S, Bianco AC (2012) Thyroid hormone deiodinases and cancer. Front Endocrinol 3:74
Debski MG, Pachucki J, Ambroziak M, Olszewski W, Bar-Andziak E (2007) Human breast cancer tissue expresses high level of type 1 5-deiodinase. Thyroid 17:3–10
Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D et al (2007) Sonic hedgehog-induced type3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104:14466–14471
Chen Z, Zhao TJ, Li J, Gao YS, Meng FG, Yan YB et al (2011) Slow skeletal muscle myosin-binding protein-C (MyBPC1) mediates recruitment of muscle-type creatine kinase (CK) to myosin. Biochem J 436:437–445
Acknowledgments
This study was funded by the Lynn Sage Cancer Research Foundation, R01CA12055501, and a generous donation from Ms. Dariel Eklund. Additional support was provided by the Amgen Inc.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong Hu and Jun Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, H., Wang, J., Gupta, A. et al. RANKL expression in normal and malignant breast tissue responds to progesterone and is up-regulated during the luteal phase. Breast Cancer Res Treat 146, 515–523 (2014). https://doi.org/10.1007/s10549-014-3049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3049-9