Abstract
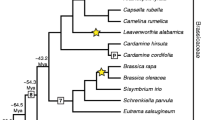
This paper examines telomeres from an evolutionary perspective. In the monocot plant order Asparagales two evolutionary switch-points in telomere sequence are known. The first occurred when the Arabidopsis-type telomere was replaced by a telomere based on a repeat motif more typical of vertebrates. The replacement is associated with telomerase activity, but the telomerase has low fidelity and this may have implications for the binding of telomeric proteins. At the second evolutionary switch-point, the telomere and its mode of synthesis are replaced by an unknown mechanism. Elsewhere in plants (Sessia, Vestia, Cestrum) and in arthropods, the telomere “typical” of the group is lost. Probably many other groups with “unusual” telomeres will be found. We question whether telomerase is indeed the original end-maintenance system and point to other candidate processes involving t-loops, t-circles, rolling circle replication and recombination. Possible evolutionary outcomes arising from the loss of telomerase activity in alternative lengthening of telomere (ALT) systems are discussed. We propose that elongation of minisatellite repeats using recombination/replication processes initially substitutes for the loss of telomerase function. Then in more established ALT groups, subtelomeric satellite repeats may replace the telomeric minisatellite repeat whilst maintaining the recombination/replication mechanisms for telomere elongation. Thereafter a retrotransposition-based end-maintenance system may become established. The influence of changing sequence motifs on the properties of the telomere cap is discussed. The DNA and protein components of telomeres should be regarded – as with any other chromosome elements – as evolving and co-evolving over time and responding to changes in the genome and to environmental stresses. We describe how telomere dysfunction, resulting in end-to-end chromosome fusions, can have a profound effect on chromosome evolution and perhaps even speciation.
Similar content being viewed by others
References
Adams SP, Hartman TP, Lim KY et al. (2001) Loss and recovery of Arabidopsis-type telomere repeat sequences 5′-(TTTAGGG)(n)-3′ in the evolution of a major radiation of flowering plants. Proc R Soc Lond B Biol Sci 268: 1541–1546.
Adams SP, Leitch IJ, Bennett MD, Leitch AR (2000) Aloe L. – a second plant family without (TTTAGGG)n telomeres. Chromosoma 109: 201–205.
Barnes SR, James AM, Jamieson G (1985) The organisation, nucleotide sequence, and chromosomal distribution of satellite DNA from Allium cepa. Chromosoma 92: 185–192.
Barton NH, Hewitt GM (1981) A chromosomal cline in the grasshopper Podisma pedestris. Evolution 35: 1008–1018.
Bedoyan JK, Lejnine S, Makarov VL, Langmore JP (1996) Condensation of rat telomere-specific nucleosomal arrays containing unusually short DNA repeats and histone H1. J Biol Chem 271: 18485–18493.
Blackburn EH, Gall JG (1978) A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 120: 33–53.
Boulton SJ, Jackson SP (1996) Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res 24: 4639–4648.
Broun P, Ganal MW, Tanksley SD (1992) Telomeric arrays display high levels of heritable polymorphism among closely related plant varieties. Proc Natl Acad Sci USA 89: 1354–1357.
Bryan TM, Marusic L, Bacchetti S, Namba M, Reddel RR (1997) The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum Mol Genet 6: 921–926.
Butlin RK (1993) Barriers to gene flow. Nature 366: 27.
Chen Q, Ijpma A, Greider CW (2001) Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol Cell Biol 21: 1819–1827.
Dudasova Z, Dudas A, Chovanec M (2004) Non-homologous end-joining factors of Saccharomyces cerevisiae. FEMS Microbiol Rev 28: 581–601.
Fajkus J, Trifonov EN (2001) Columnar packing of telomeric nucleosomes. Biochem Biophys Res Commun 280: 961–963.
Fajkus J, Vyskot B, Bezdek M (1992) Changes in chromatin structure due to hypomethylation induced with 5-azacytidine or dL-ethionine. FEBS Lett 314: 13–16.
Fajkus J, Kovarik A, Kralovics R, Bezdek M (1995) Organization of telomeric and subtelomeric chromatin in the higher plant Nicotiana tabacum. Mol Gen Genet 247: 633–638.
Fajkus J, Novotná M, Ptáček J (2002) Analysis of chromosome termini in potato varieties. Rostl Výroba 48: 477–479.
Fajkus J, Sykorova E, Leitch AR (2005) Techniques in plant telomere biology. Biotechniques 38: 233–243.
Fay MF, Rudall PJ, Sullivan S et al. (2000) Phylogentic studies of Asparagales based on four plastid regions. In: Wilson KL, Morrison DA eds., Monocots: Systematics and Evolution. Melbourne: CSIRO, pp. 360–371.
Featherstone C, Jackson SP (1998) DNA repair: the Nijmegen breakage syndrome protein. Curr Biol 8: R622–625.
Fitzgerald MS, Riha K, Gao F, Ren S, McKnight TD, Shippen DE (1999) Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc Natl Acad Sci USA 96: 14813–14818.
Fransz PF, Alonso-Blanco C, Liharska TB, Peeters AJ, Zabel P, de Jong JH (1996) High-resolution physical mapping in Arabidopsis thaliana and tomato by fluorescence in situ hybridization to extended DNA fibres. Plant J 9: 421–430.
Frydrychova R, Marec F (2002) Repeated losses of TTAGG telomere repeats in evolution of beetles (Coleoptera). Genetica 115: 179–187.
Frydrychova R, Grossmann P, Trubac P, Vitkova M, Marec F (2004) Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 47: 163–178.
Ganal MW, Lapitan NL, Tanksley SD (1991) Macrostructure of the tomato telomeres. Plant Cell 3: 87–94.
Garcia-Cao M, O’Sullivan R, Peters AH, Jenuwein T, Blasco MA (2004) Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet 36: 94–99.
Gazdova B, Siroky J, Fajkus J et al. (1995) Characterization of a new family of tobacco highly repetitive DNA, GRS, specific for the Nicotiana tomentosiformis genomic component. Chromosome Res 3: 245–254.
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43: 405–413.
Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51: 887–898.
Hartmann N, Scherthan H (2004) Characterization of ancestral chromosome fusion points in the Indian muntjac deer. Chromosoma 112: 213–220.
Hauffe HC, Panithanarak T, Dallas JF, Pialek J, Gunduz I, Searle JB (2004) The tobacco mouse and its relatives: a “tail” of coat colors, chromosomes, hybridization and speciation. Cytogenet Genome Res 105: 395–405.
Hewitt GM, Nichols RA, Barton NH (1987) Homogamy in a hybrid zone in the alpine grasshopper Podisma pedestris. Heredity 59: 457–466.
Ioshikhes I, Bolshoy A, Trifonov EN (1992) Preferred positions of AA and TT dinucleotides in aligned nucleosomal DNA sequences. J Biomol Struct Dyn 9: 1111–1117.
Ioshikhes I, Bolshoy A, Derenshteyn K, Borodovsky M, Trifonov EN (1996) Nucleosome DNA sequence pattern revealed by multiple alignment of experimentally mapped sequences. J Mol Biol 262: 129–139.
King M (1993) Species Evolution. The role of chromosome change, Cambridge: Cambridge University Press.
Kovařík A, Fajkus J, Koukalová BE, Bezděk M (1996) Species-specific evolution of telomeric and rDNA repeats in the tobacco composite genome. Theor Appl Genet 92: 1108–1111.
Kralovics R, Fajkus J, Kovarik A, Bezdek M (1995) DNA curvature of the tobacco GRS repetitive sequence family and its relation to nucleosome positioning. J Biomol Struct Dyn 12: 1103–1119.
Kuchar M, Fajkus J (2004) Interactions of putative telomere-binding proteins in Arabidopsis thaliana: identification of functional TRF2 homolog in plants. FEBS Lett 578: 311–315.
Lee C, Sasi R, Lin CC (1993) Interstitial localization of telomeric DNA sequences in the Indian muntjac chromosomes: further evidence for tandem chromosome fusions in the karyotypic evolution of the Asian muntjacs. Cytogenet Cell Genet 63: 156–159.
Lei M, Podell ER, Cech TR (2004) Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol 11: 1223–1229.
Lejnine S, Makarov VL, Langmore JP (1995) Conserved nucleoprotein structure at the ends of vertebrate and invertebrate chromosomes. Proc Natl Acad Sci USA 92: 2393–2397.
Levin DA (2002) The Role of Chromosomal Change in Plant Evolution. New York: Oxford University Press.
Liu D, Safari A, O’Connor MS et al. (2004) PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6: 673–680.
Lopez CC, Rodriguez E, Diez JL, Edstrom J, Morcillo G (1999) Histochemical localization of reverse transcriptase in polytene chromosomes of chironomids. Chromosoma 108: 302–307.
Louis EJ (2002) Are Drosophila telomeres an exception or the rule? Genome Biol 3: Review S0007.
Makarov VL, Lejnine S, Bedoyan J, Langmore JP (1993) Nucleosomal organization of telomere-specific chromatin in rat. Cell 73: 775–787.
McClintock B (1938) The fusion of broken chromosome ends of sister half-chromatids following chromatid breakage at meiotic anaphases. Missouri Agric Exp Station Res Bull 290: 1–48.
McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26: 234–282.
Muller HJ (1938) The remaking of chromosomes. Collecting Net. 13: 181–195, 198.
Nosek J, Rycovska A, Makhov AM, Griffith JD, Tomaska L (2005) Amplification of telomeric arrays via rolling-circle mechanism. J Biol Chem 280: 10840–10845.
K Oguchi H Liu K Tamura H Takahashi (1999) ArticleTitleMolecular cloning and characterization of AtTERT, a telomerase reverse transcriptase homolog in Arabidopsis thaliana FEBS Lett 457 465–469 Occurrence Handle10.1016/S0014-5793(99)01083-2 Occurrence Handle10471830
Pearce SR, Pich U, Harrison G, Flavell AJ et al. (1996) The Ty1-copia group retrotransposons of Allium cepa are distributed throughout the chromosomes but are enriched in the terminal heterochromatin. Chromosome Res 4: 357–364.
Pich U, Schubert I (1998) Terminal heterochromatin and alternative telomeric sequences in Allium cepa. Chromosome Res 6: 315–321.
Pich U, Fritsch R, Schubert I (1996a) Closely related Allium species (Alliaceae) share a very similar satellite sequence. Plant Syst Evol 202: 255–264.
Pich U, Fuchs J, Schubert I (1996b) How do Alliaceae stabilize their chromosome ends in the absence of TTTAGGG sequences? Chromosome Res 4: 207–213.
Porter G, Westmoreland J, Priebe S, Resnick MA (1996) Homologous and homeologous intermolecular gene conversion are not differentially affected by mutations in the DNA damage or the mismatch repair genes RAD1, RAD50, RAD51, RAD52, RAD54, PMS1 and MSH2. Genetics 143: 755–767.
Puizina J, Weiss-Schneeweiss H, Pedrosa-Harand A et al. (2003) Karyotype analysis in Hyacinthella dalmatica (Hyacinthaceae) reveals vertebrate-type telomere repeats at the chromosome ends. Genome 46: 1070–1076.
Puizina J, Siroky J, Mokros P, Schweizer D, Riha K (2004) Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell 16: 1968–1978.
K Ríha DE Shippen (2003) ArticleTitleKu is required for telomeric C-rich strand maintenance but not for end-to-end chromosome fusions in Arabidopsis Proc Natl Acad Sci USA 100 611–615 Occurrence Handle10.1073/pnas.0236128100 Occurrence Handle12511598
Ríha K, McKnight TD, Griffing LR, Shippen DE (2001) Living with genome instability: plant responses to telomere dysfunction. Science 291: 1797–1800.
Richards EJ, Ausubel FM (1988) Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell 53: 127–136.
Rosen M, Edstrom J (2000) DNA structures common for chironomid telomeres terminating with complex repeats. Insect Mol Biol 9: 341–347.
Rossetti L, Cacchione S, Fua M, Savino M (1998) Nucleosome assembly on telomeric sequences. Biochemistry 37: 6727–6737.
Rotkova G, Sklenickova M, Dvorackova M, Sykorova E, Leitch AR, Fajkus J (2004) An evolutionary change in telomere sequence motif within the plant section Asparagales had significance for telomere nucleoprotein complexes. Cytogenet Genome Res 107: 132–138.
Sadaie M, Naito T, Ishikawa F (2003) Stable inheritance of telomere chromatin structure and function in the absence of telomeric repeats. Genes Dev 17: 2271–2282.
Sahara K, Marec F, Traut W (1999) TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res 7: 449–460.
Sasaki T, Fujiwara H (2000) Detection and distribution patterns of telomerase activity in insects. Eur J Biochem 267: 3025–3031.
Schrumpfova P, Kuchar M, Mikova G, Skrisovska L, Kubicarova T, Fajkus J (2004) Characterization of two Arabidopsis thaliana myb-like proteins showing affinity to telomeric DNA sequence. Genome 47: 316–324.
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92: 143–148.
Siroky J, Zluvova J, Riha K, Shippen DE, Vyskot B (2003) Rearrangements of ribosomal DNA clusters in late generation telomerase-deficient Arabidopsis. Chromosoma 112: 116–123.
Sykorova E, Fajkus J, Ito M, Fukui K (2001) Transition between two forms of heterochromatin at plant subtelomeres. Chromosome Res 9: 309–323.
Sykorova E, Lim KY, Fajkus J, Leitch AR (2003a) The signature of the Cestrum genome suggests an evolutionary response to the loss of (TTTAGGG)n telomeres. Chromosoma 112: 164–172.
Sykorova E, Lim KY, Chase MW, Knapp S, Leitch IJ, Leitch AR, Fajkus J (2003b) The absence of Arabidopsis-type telomeres in Cestrum and closely related genera Vestia and Sessea (Solanaceae): first evidence from eudicots. Plant J 34: 283–291.
Sykorova E, Lim KY, Kunicka Z et al. (2003c) Telomere variability in the monocotyledonous plant order Asparagales. Proc R Soc Lond B Biol Sci 270: 1893–1904.
Teng SC, Zakian VA (1999) Telomere–telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol 19: 8083–8093.
Tomaska L, Nosek J, Makhov AM, Pastorakova A, Griffith JD (2000) Extragenomic double-stranded DNA circles in yeast with linear mitochondrial genomes: potential involvement in telomere maintenance. Nucleic Acids Res 28: 4479–4487.
Tomaska L, McEachern MJ, Nosek J (2004) Alternatives to telomerase: keeping linear chromosomes via telomeric circles. FEBS Lett 567: 142–146.
Tommerup H, Dousmanis A, de Lange T (1994) Unusual chromatin in human telomeres. Mol Cell Biol 14: 5777–5785.
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413.
Vershinin AV, Heslop-Harrison JS (1998) Comparative analysis of the nucleosomal structure of rye, wheat and their relatives. Plant Mol Biol 36: 149–161.
Vitkova M, Kral J, Traut W, Zrzavy J, Marec F (2005) The evolutionary origin of insect telomeric repeats, (TTAGG)n. Chromosome Res 13: 145–156.
Weiss H, Scherthan H (2002) Aloe spp. – plants with vertebrate-like telomeric sequences. Chromosome Res 10: 155–164.
Weiss-Schneeweiss H, Riha K, Jang CG, Puizina J, Scherthan H, Schweizer D (2004) Chromosome termini of the monocot plant Othocallis siberica are maintained by telomerase, which specifically synthesises vertebrate-type telomere sequences. Plant J 37: 484–493.
West CE, Waterworth WM, Sunderland PA, Bray CM (2004) Arabidopsis DNA double-strand break repair pathways. Biochem Soc Trans 32: 964–966.
White MJD (1973) Animal Cytology and Evolution. London: Cambridge University Press.
Yang F, Carter NP, Shi L, Ferguson-Smith MA (1995) A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103: 642–652.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fajkus, J., Sýkorová, E. & Leitch, A.R. Telomeres in evolution and evolution of telomeres. Chromosome Res 13, 469–479 (2005). https://doi.org/10.1007/s10577-005-0997-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-005-0997-2