Abstract
Background and Aim
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder where a high frequency of gastrointestinal disturbance (e.g., constipation and diarrhea) is reported. As large bowel fermentation products can have beneficial or detrimental effects on health, these were measured in feces of children with and without ASD to examine whether there is an underlying disturbance in fermentation processes in the disorder.
Methods
Fecal samples (48 h) were collected from children with ASD (n = 23), and without ASD (n = 31) of similar age. Concentrations of short chain fatty acids, phenols and ammonia were measured.
Results
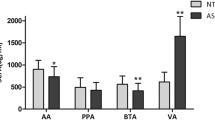
Fecal total short chain fatty acid concentrations were significantly higher in children with ASD compared to controls (136.6 ± 8.7 vs. 111.1 ± 6.6 mmol/kg). Moreover, when concentrations of fecal acetic, butyric, isobutyric, valeric, isovaleric and caproic acids were measured, all were significantly higher in children with ASD compared with controls except for caproic acid. The concentration of fecal ammonia was also significantly greater in ASD participants than controls (42.7 ± 3.3 vs. 32.3 ± 1.9 mmol/kg). Fecal phenol levels and pH did not differ between groups. Macronutrient intake, as determined from dietary records kept by caregivers, also did not differ significantly between study groups.
Conclusions
Our results suggest fermentation processes or utilization of fermentation products may be altered in children with ASD compared to children without ASD.
Similar content being viewed by others
References
Eapen V. Genetic basis of autism: is there a way forward? Curr Opin Psychiatr. 2011;24:226–236.
Buie T, Campbell DB, Fuchs Iii GJ, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125:S1–S18.
Wang L, Angley MT, Gerber JP, et al. Is urinary indolyl-3-acryloylglycine a biomarker for autism with gastrointestinal symptoms? Biomarkers. 2009;14:596–603.
Campbell DB, Buie TM, Winter H, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024.
Wang L, Christophersen CT, Sorich MJ, et al. Low relative abundances of the mucolytic bacterium akkermansia muciniphila and bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011;77:6718–6721.
Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453.
Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064.
Nowak A, Libudzisz Z. Influence of phenol, p-cresol and indole on growth and survival of intestinal lactic acid bacteria. Anaerobe. 2006;12:80–84.
Meletis CD, Zabriskie N. Supporting gastrointestinal health with nutritional therapy. Altern Complement Ther. 2008;14:132–138.
Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE. 2011;6:e24585.
Smith RA, Farnworth H, Wright B, Allgar V. Are there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders?: a case control study. Autism. 2009;13:343–355.
Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–132.
King RA, May BL, Davies DA, Bird AR. Measurement of phenol and p-cresol in urine and feces using vacuum microdistillation and high-performance liquid chromatography. Anal Biochem. 2009;384:27–33.
McOrist AL, Abell GCJ, Cooke C, Nyland K. Bacterial population dynamics and faecal short-chain fatty acid (SCFA) concentrations in healthy humans. Brit J Nutr. 2008;100:138–146.
MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–169.
MacFabe DF, Rodraguez-Capote K, Hoffman JE, et al. A novel rodent model of autism: Intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotechnol. 2008;4:146–166.
Wong JMW, De Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243.
Tjellstrom B, Stenhammar L, Hogberg L, et al. Gut microflora associated characteristics in children with celiac disease. Am J Gastroenterol. 2005;100:2784–2788.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336.
Maslowski KM, Vieira AT, Ng A, Kranich J, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286.
D’Eufemia P, Celli M, Finocchiaro R, et al. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85:1076–1079.
de Magistris L, Familiari V, Pascotto A, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastr Nutr. 2010;51:418–424.
Messahel S, Pheasant AE, Pall H, et al. Urinary levels of neopterin and biopterin in autism. Neurosci Lett. 1998;241:17–20.
van Nuenen MH, Venema K, van der Woude JC, Kuipers EJ. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:485–491.
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism—comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22.
Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial [beta]-oxidation by long chain acyl-CoA dehydrogenase. Med Hypotheses. 2004;62:970–975.
Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–623.
Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259–279.
Altieri L, Neri C, Sacco R, et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16:9.
Yap IK, Angley M, Veselkov KA, et al. Urinary metabolic phenotyping differentiates children with autism, from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004.
Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci USA. 2009;106:14728–14733.
Birkett AM, Jones GP, de Silva AM, Young GP, Muir JG. Dietary intake and faecal excretion of carbohydrate by Australians: importance of achieving stool weights greater than 150 g to improve faecal markers relevant to colon cancer risk. Euro J Clin Nutr. 1997;51:625–632.
Hopkins MJ, Sharp R, Macfarlane GT. Variation in human intestinal microbiota with age. Dig Liver Dis. 2002;34:S12–S18.
Whiteley P, Todd L, Carr K, Shattock P. Gender ratios in autism, Asperger syndrome and autism spectrum disorder. Autism Insights. 2010;2:17–24.
McOrist AL, Miller RB, Bird AR, et al. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141:883–889.
Bird AR, Conlon MA, Christophersen CT, Topping DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes. 2010;1:423–431.
Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547.
Acknowledgments
This research was funded by The Australian Rotary Health Research Fund. We wish to express our gratitude to the participating children and their parents. We would also like to acknowledge Dr Richard Couper, pediatric gastroenterologist, for medical advice; Mrs. Rosalind Miller for statistical assistance; and Emma Watson, Ben Scherer, Bruce May and Rhys Bushell for technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Christophersen, C.T., Sorich, M.J. et al. Elevated Fecal Short Chain Fatty Acid and Ammonia Concentrations in Children with Autism Spectrum Disorder. Dig Dis Sci 57, 2096–2102 (2012). https://doi.org/10.1007/s10620-012-2167-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2167-7