Summary
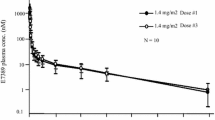
The aim of this study was to determine the maximum tolerated dose, dose-limiting toxicities, and pharmacokinetic profile of E7107 in patients with advanced solid tumors. Patients in this phase I, open-label, single-arm, dose-escalation study had metastatic or locally advanced solid tumors and received E7107 as a 30-minute intravenous infusion at doses of 0.6, 1.2, 1.8, 2.4, 3.2, 4.3, and 5.7 mg/m2. Twenty-six patients were enrolled in the study. At 5.7 mg/m2, two patients experienced dose-limiting toxicities including diarrhea, vomiting, dehydration, and myocardial infarction on Days 1–3 following E7107 administration. Three additional patients were recruited at the lower dose and all six patients tolerated E7107 4.3 mg/m2 with no dose-limiting toxicities. The maximum tolerated dose of E7107 was therefore 4.3 mg/m2. The most common drug-related adverse events were nausea, vomiting, and diarrhea. Vision loss was experienced by two patients at Cycles 2 and 7, each patient receiving 3.2 mg/m2 and 4.3 mg/m2, respectively. This resulted in the study being put on clinical hold. Pharmacokinetic analysis showed that E7107 was rapidly distributed with a moderate elimination half-life (6–13 h) and high clearance. Exposure to E7107 was dose-related. The best tumor response was stable disease in eight patients. E7107 is a unique first-in-class molecule. The incidence of two cases of vision loss probably related to E7107 led to study discontinuation.


Similar content being viewed by others
References
Folco EG, Coil KE, Reed R (2011) The anti-tumor drug E7107 reveals an essential role for SF3b in remodeling U2 snRNP to expose the branch point-binding region. Genes Dev 25:440–444. doi:10.1101/gad.2009411
Kotake Y, Sagane K, Owa T (2007) Splicing factor SF3b as the molecular target of novel antitumor agent pladienolide [abstract 8211]. In: Program and abstracts of the Twelfth Annual Meeting of the RNA Society, Madison, WI, May 29–June 3
van Alphen RJ, Wiemer EA, Burger H, Eskens FA (2009) The spliceosome as target for anticancer treatment. Br J Cancer 100:228–232. doi:10.1038/sj.bjc.6604801
Uenaka T, Iwata M, Ozawa Y, Shimizu H, Kotake Y, Mizui Y, Yoshimatsu K, Asada M (2004) E7107, a new 7-urethane derivative of pladienolide D: correlation of the profile of cell cycle regulatory molecules with tumor cells’ response [abstract 2991]. Proc Am Assoc Cancer Res 45:691
Mizui Y, Sakai T, Iwata M, Uenaka T, Okamoto K, Shimizu H, Yamori T, Yoshimatsu K, Asada M (2004) Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. III. In vitro and in vivo antitumor activities. J Antibiot (Tokyo) 57:188–196. doi:10.7164/antibiotics.57.188
Iwata M, Ozawa Y, Uenaka T, Shimizu H, Niijima J, Kanada RM, Fukuda Y, Nagai M, Kotake Y, Yoshida M, Tsuchida T, Mizui Y, Yoshimatsu K, Asada M (2004) E7107, a new 7-urethane derivative of pladienolide D, displays curative effect against several human tumor xenografts [abstract 2989]. Proc Am Assoc Cancer Res 45:691
Niijima J, Kotake Y, Kanada RM, Nagai M, Fukuda Y, Sakai T, Ishihara H, Yoshida M, Tsuchida T, Iwata M, Uenaka T, Mizui Y, Abe S, Yoshimatsu K, Asada M (2004) E7107, a new 7-urethane derivative of pladienolide D: Discovery of a novel antitumor agent [abstract 2988]. Proc Am Assoc Cancer Res 45:691
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147. doi:10.1093/jnci/89.15.1138
Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS (2000) Phase I clinical trial design in cancer drug development. J Clin Oncol 18:684–692
Affymetrix Inc. (2005) Technical note: GeneChip® exon array design. http://media.affymetrix.com/support/technical/technotes/exon_array_design_technote.pdf. Accessed 25 July 2013
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216. doi:10.1093/jnci/92.3.205
McKie AB, McHale JC, Keen TJ, Tarttelin EE, Goliath R, van Lith-Verhoeven JJ, Greenberg J, Ramesar RS, Hoyng CB, Cremers FP, Mackey DA, Bhattacharya SS, Bird AC, Markham AF, Inglehearn CF (2001) Mutations in the pre-mRNA splicing factor gene PRPC8 in autosomal dominant retinitis pigmentosa (RP13). Hum Mol Genet 10:1555–1562. doi:10.1093/hmg/10.15.1555
Vithana EN, Abu-Safieh L, Allen MJ, Carey A, Papaioannou M, Chakarova C, Al-Maghtheh M, Ebenezer ND, Willis C, Moore AT, Bird AC, Hunt DM, Bhattacharya SS (2001) A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11). Mol Cell 8:375–381. doi:10.1016/S1097-2765(01)00305-7
Chakarova CF, Hims MM, Bolz H, Abu-Safieh L, Patel RJ, Papaioannou MG, Inglehearn CF, Keen TJ, Willis C, Moore AT, Rosenberg T, Webster AR, Bird AC, Gal A, Hunt D, Vithana EN, Bhattacharya SS (2002) Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum Mol Genet 11:87–92. doi:10.1093/hmg/11.1.87
Deery EC, Vithana EN, Newbold RJ, Gallon VA, Bhattacharya SS, Warren MJ, Hunt DM, Wilkie SE (2002) Disease mechanism for retinitis pigmentosa (RP11) caused by mutations in the splicing factor gene PRPF31. Hum Mol Genet 11:3209–3219. doi:10.1093/hmg/11.25.3209
Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M, Fangazio M, Vaisitti T, Monti S, Chiaretti S, Guarini A, Del Giudice I, Cerri M, Cresta S, Deambrogi C, Gargiulo E, Gattei V, Forconi F, Bertoni F, Deaglio S, Rabadan R, Pasqualucci L, Foá R, Dalla-Favera R, Gaidano G (2011) Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood 118:6904–6908. doi:10.1182/blood-2011-08-373159
Patnaik MM, Lasho TL, Hodnefield JM, Knudson RA, Ketterling RP, Garcia-Manero G, Steensma DP, Pardanani A, Hanson CA, Tefferi A (2012) SF3B1 mutations are prevalent in myelodysplastic syndromes with ring sideroblasts but do not hold independent prognostic value. Blood 119:569–572. doi:10.1182/blood-2011-09-377994
Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, Pellagatti A, Wainscoat JS, Hellstrom-Lindberg E, Gambacorti-Passerini C, Godfrey AL, Rapado I, Cvejic A, Rance R, McGee C, Ellis P, Mudie LJ, Stephens PJ, McLaren S, Massie CE, Tarpey PS, Varela I, Nik-Zainal S, Davies HR, Shlien A, Jones D, Raine K, Hinton J, Butler AP, Teague JW, Baxter EJ, Score J, Galli A, Della Porta MG, Travaglino E, Groves M, Tauro S, Munshi NC, Anderson KC, El-Naggar A, Fischer A, Mustonen V, Warren AJ, Cross NC, Green AR, Futreal PA, Stratton MR, Campbell PJ, Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium (2011) Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med 365:1384–1395. doi:10.1056/NEJMoa1103283
Visconte V, Makishima H, Jankowska A, Szpurka H, Traina F, Jerez A, O’Keefe C, Rogers HJ, Sekeres MA, Maciejewski JP, Tiu RV (2012) SF3B1, a splicing factor is frequently mutated in refractory anemia with ring sideroblasts. Leukemia 26:542–545. doi:10.1038/leu.2011.232
Damm F, Thol F, Kosmider O, Kade S, Loffeld P, Dreyfus F, Stamatoullas-Bastard A, Tanguy-Schmidt A, Beyne-Rauzy O, de Botton S, Guerci-Bresler A, Göhring G, Schlegelberger B, Ganser A, Bernard OA, Fontenay M, Heuser M (2012) SF3B1 mutations in myelodysplastic syndromes: clinical associations and prognostic implications. Leukemia 26:1137–1140. doi:10.1038/leu.2011.321
Acknowledgments
We gratefully acknowledge the participating patients, their families, and study investigators for their invaluable contribution. Editorial support was provided by Deborah McGregor, PhD, of Complete Medical Communications, funded by Eisai Inc., and Jo Ann of the Department of Investigational Cancer Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston, Texas. This study was funded by Eisai Inc.
Authors’ contributions
All authors were involved in the writing, review, and/or revision of the manuscript, and have approved the manuscript for submission. D. Hong and P. LoRusso were principal investigators for the study and were involved in the development of the methodology, the acquisition of data, and the analysis and interpretation of the results. R. Kurzrock, A. Naing, J. Wheler, G. Falchook, J. Schiffman, N. Faulkner, and M. J. Pilat were involved in the acquisition of data. J. O’Brien was involved in the development of the methodology, and the analysis and interpretation of the results.
Financial support
This study was funded by Eisai Inc.
Conflicts of interest
D. S. Hong received research grant funding from Eisai for this study. J. O’Brien is affiliated with Eisai Inc., NJ, USA. The remaining authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
The submission is original work not previously published in any substantial part, and is not under consideration for publication elsewhere.
The manuscript has been read and approved for submission by all authors.
Rights and permissions
About this article
Cite this article
Hong, D.S., Kurzrock, R., Naing, A. et al. A phase I, open-label, single-arm, dose-escalation study of E7107, a precursor messenger ribonucleic acid (pre-mRNA) splicesome inhibitor administered intravenously on days 1 and 8 every 21 days to patients with solid tumors. Invest New Drugs 32, 436–444 (2014). https://doi.org/10.1007/s10637-013-0046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0046-5