Abstract
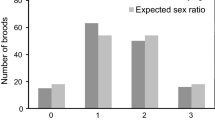
Wild steelhead (Oncorhynchus mykiss) returning to the Hood River in Oregon, USA, show a strongly female-biased sex ratio (average of 63 % female from brood years 1992 to 2004) while first-generation hatchery steelhead (created using local, wild broodstock) display an even sex ratio (51 % female). We considered four hypotheses to explain the difference in sex ratio between populations. First, it is well established that wild male O. mykiss adopt the resident (non-anadromous) life history phenotype at a higher rate than do females, and that the propensity to become resident is under genetic and environmental influence. Therefore, the simplest explanation for the difference in sex ratio between anadromous wild and hatchery fish is that hatchery males adopt the resident life history at a lower rate than do wild males. However, alternative explanations include (1) sex reversal of female hatchery fish to phenotypic males, (2) selection against hatchery females or (3) selection against wild males. The possibility of sex reversal in the hatchery was of particular interest given increased temperature has been shown to skew sex ratios to a male bias. Using a Y-chromosome specific marker (OmyY1 locus) and samples of wild and hatchery fish from various life stages, we were able to reject alternative hypotheses 1, 2 and 3. Hatchery fish were sampled at three life stages (onset of exogenous feeding, 1-year of age and returning adult). Phenotypic and chromosomal sex matched in all 1-year old and adult hatchery samples. Therefore, we see no evidence for sex reversal in the hatchery population. Furthermore, hatchery fish at all three life stages exhibited a 50:50 chromosomal (OmyY1 marker) sex ratio. Therefore, selection against hatchery females while in captivity or after release can be ruled out. The chromosomal sex ratio in a sample of wild smolts was female-biased and matched the sex ratio in returning adults from the same cohort. Therefore, we can also rule out selection against wild males at sea. Given no evidence for sex reversal or selection against either sex it seems most plausible that the greater female bias in wild, compared to hatchery, steelhead from the Hood River results from differential life history expression in males. Wild males appear to become resident (non-anadromous) at a higher rate than do hatchery males.
Similar content being viewed by others
References
Andersen BC (1983) Fish populations of Carnation Creek and other Barkley Sound streams 1970–1980. Can Data Rept Fish Aquat Sci No. 415, 159 p
Araki H, Ardren WR, Olsen E et al (2007a) Reproductive success of captive-bred steelhead trout in the wild: evaluation of three hatchery programs in the Hood River. Conserv Biol 21:181–190. doi:10.1111/j.1523-1739.2006.00564.x
Araki H, Cooper B, Blouin MS (2007b) Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 318:100–103. doi:10.1126/science.1145621
Azuma T, Takeda K, Doi T et al (2004) The influence of temperature on sex determination in sockeye salmon Oncorhynchus nerka. Aquaculture 234:461–473. doi:10.1016/j.aquaculture.2003.11.023
Brunelli JP, Wertzler KJ, Sundin K, Thorgaard GH (2008) Y-specific sequences and polymorphisms in rainbow trout and Chinook salmon. Genome 51:739–748. doi:10.1139/G08-060
Christie MR, Marine ML, Blouin MS (2011) Who are the missing parents? Grandparentage analysis identifies multiple sources of gene flow into a wild population. Mol Ecol 20:1263–1276. doi:10.1111/j.1365-294X.2010.04994.x
Cole KS (2010) Gonad Development in Hermaphroditic Gobies. Reproduction and sexuality in marine fishes. University of California Press, Berkeley, pp 165–202
D’cotta H, Fostier A, Guiguen Y et al (2001) Aromatase plays a key role during normal and temperature-induced sex differentiation of tilapia Oreochromis niloticus. Mol Reprod Dev 59:265–276
Fenske M, Segner H (2004) Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat Toxicol 67:105–126
Hayes SA, Bond MH, Hanson CV, MacFarlane RB (2004) Interactions between endangered wild and hatchery salmonids: can the pitfalls of artificial propagation be avoided in small coastal streams? J Fish Biol 65:101–121
Hecht BC, Campbell NR, Holecek DE, Narum SR (2013) Genome-wide association reveals genetic basis for the propensity to migrate in wild populations of rainbow and steelhead trout. Mol Ecol 22:3061–3076. doi:10.1111/mec.12082
Holtby LB, Healey MC (1990) Sex-specific life history tactics and risk-taking in coho salmon. Ecology 71:678–690
Kendall NW, Quinn TP (2013) Size-selective fishing affects sex ratios and the opportunity for sexual selection in Alaskan sockeye salmon Oncorhynchus nerka. Oikos 122:411–420. doi:10.1111/j.1600-0706.2012.20319.x
Kostow KE (2004) Differences in juvenile phenotypes and survival between hatchery stocks and a natural population provide evidence for modified selection due to captive breeding. Can J Fish Aquat Sci 61:577–589. doi:10.1139/f04-019
Kroon FJ, Munday PL, Westcott DA et al (2005) Aromatase pathway mediates sex change in each direction. Proc R Soc B Biol Sci 272:1399–1405
Leider SA, Chilcote MW, Loch JJ (1986) Comparative life history characteristics of hatchery and wild steelhead trout (Salmo gairdneri) of summer and winter races in the Kalama River, Washington. Can J Fish Aquat Sci 43:1398–1409
Magerhans A, Hörstgen-Schwark G (2010) Selection experiments to alter the sex ratio in rainbow trout (Oncorhynchus mykiss) by means of temperature treatment. Aquaculture 306:63–67
Milot E, Perrier C, Papillon L et al (2013) Reduced fitness of Atlantic salmon released in the wild after one generation of captive breeding. Evol Appl 6:472–485. doi:10.1111/eva.12028
Narver DW (1969) Age and size of steelhead trout in the Babine River, British Columbia. J Fish Board Can 26:2754–2760
Ohms H (2012) The influence of sex, migration distance, and latitude on expression of anadromy in Oncorhynchus mykiss. M.Sc. thesis, Oregon State University
Piferrer F, Zanuy S, Carrillo M et al (1994) Brief treatment with an aromatase inhibitor during sex differentiation causes chromosomally female salmon to develop as normal, functional males. J Exp Zool 270:255–262. doi:10.1002/jez.1402700304
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout. American Fisheries Society, Bethesda
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reagan RE (2010) Hood River and Pelton Ladder Evaluation Studies. Annual report 2009–2010 of the Oregon Department of Fish and Wildlife. Oregon Department of Fish and Wildlife, Portland
Rundio DE, Williams TH, Pearse DE, Lindley ST (2012) Male-biased sex ratio of nonanadromous Oncorhynchus mykiss in a partially migratory population in California. Ecol Freshw Fish 21:293–299. doi:10.1111/j.1600-0633.2011.00547.x
Seamons TR, Bentzen P, Quinn TP (2004) The mating system of steelhead, Oncorhynchus mykiss, inferred by molecular analysis of parents and progeny. Environ Biol Fish 69:333–344
Spidle AP, Quinn TP, Bentzen P (1998) Sex-biased marine survival and growth in a population of coho salmon. J Fish Biol 52:907–915
Tamate T, Maekawa K (2004) Female-biased mortality rate and sexual size dimorphism of migratory masu salmon, Oncorhynchus masou. Ecol Freshw Fish 13:96–103
Uchida D, Yamashita M, Kitano T, Iguchi T (2004) An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A: Mol Integr Physiol 137:11–20
Vizziano D, Baron D, Randuineau G et al (2008) Rainbow trout gonadal masculinization induced by inhibition of estrogen synthesis is more physiological than masculinization induced by androgen supplementation. Biol Reprod 78:939–946. doi:10.1095/biolreprod.107.065961
Watts M, Pankhurst NW, King HR (2004) Maintenance of Atlantic salmon (Salmo salar) at elevated temperature inhibits cytochrome P450 aromatase activity in isolated ovarian follicles. Gen Comp Endocrinol 135:381–390. doi:10.1016/j.ygcen.2003.11.004
Williamson KS, May B (2005) Inheritance studies implicate a genetic mechanism for apparent sex reversal in Chinook salmon. Trans Am Fish Soc 134:1253–1261
Williamson KS, Phillips R, May B (2008) Characterization of a chromosomal rearrangement responsible for producing “apparent” XY-female fall-run Chinook salmon in California. J Hered 99:483–490
Withler IL (1966) Variability in life history characteristics of steelhead trout (Salmo gairdneri) along the Pacific coast of North America. J Fish Board Can 23:365–393
Yano A, Nicol B, Jouanno E et al (2013) The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol Appl 6:486–496
Acknowledgments
We would like to thank David Noakes for insightful discussion that led to the initiation of the project. We would also like to thank Jim Gidley and Albert Santos from the Parkdale Fish Facility and the entire crew at Oak Springs hatchery for all their help in producing fish for the project. We also thank R. French, P. Simpson, and all the Oregon Department of Fish and Wildlife staff that collected and provided tissue samples for this project. The Oregon State Institutional Animal Care and Use Committee approved this study. This work was supported by a grant from the Bonneville Power Administration to M.S. Blouin.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 140 kb)
Rights and permissions
About this article
Cite this article
Thompson, N.F., Cole, K.S., McMahon, L.A. et al. Sex reversal, selection against hatchery females or wild males does not explain differences in sex ratio between first generation hatchery and wild steelhead, Oncorhynchus mykiss . Environ Biol Fish 98, 113–120 (2015). https://doi.org/10.1007/s10641-014-0240-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0240-0