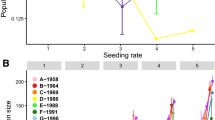
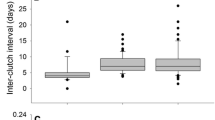
Abstract
Crowding is known to have a major influence on reproduction in the freshwater microcrustacean Daphnia pulex. We analyzed reproductive output of six different D. pulex genotypes under two different density regimes in the laboratory. Four of these genotypes reproduce via obligate parthenogenesis, allowing thorough analysis of the life history strategies of some asexual lines. Among 30,109 neonate offspring and 1041 resting egg ephippia collected, several trends were evident. Crowding induced increased resting egg production and reduced neonate offspring production among all genotypes. Offspring sex ratios grew more male-biased with maternal age. The extent, but not direction, of each of these trends varied among genotypes. Offspring sex ratios, and the very direction in which they changed in response to crowding, differed significantly among genotypes with some genotypes producing more and others fewer males in response to crowding. Obligately parthenogenetic genotypes seemed to respond to the crowding stimulus in similar ways as the facultatively parthenogenetic genotypes, as expected from the sexual origins of their genomes. The inter-genotype variation in life-history traits observed in this and other investigations calls into question the common practice of extrapolating results from a single Daphnia genotype to an entire species. Our findings are considered in the context of other research in the field of environmental influences on Daphnia reproduction with a review of representative literature.
Similar content being viewed by others
References
Alekseev V. and Lampert W. (2001). Maternal control of resting-egg production in Daphnia. Nature 414: 899–901
Antunes S. C., Castro B. B. and Gonçalves F. (2003). Chronic responses of different clones of Daphnia longispina (field and ephippia) to different food levels. Acta Oecologica 24: S325–S332
Baird D. J., Barber I., Bradley M., Soares A. M. V. M. and Calow P. (1991). A comparative study of genotype sensitivity to acute toxic stress using clones of Daphnia magna Straus. Ecotoxicology and Environmental Safety 21: 257–265
Banta A. M. and Brown L. A. (1929). Control of sex in Cladocera. I. crowding the mothers as a means of controlling male production. Physiological Zoölogy 2: 80–92
Barker D. M. and Hebert P. D. N. (1986). Secondary sex ratio of the cyclic parthenogen Daphnia magna (Crustacea: Cladocera) in the Canadian Arctic. Canadian Journal of Zoology 64: 1137–1143
Bensch S., Westerdahl H., Hansson B. and Hasselquist D. (1999). Do females adjust the sex of their offspring in relation to the breeding sex ratio?. Journal of Evolutionary Biology 12: 1104–1109
Berg L. M., Pálsson S. and Lascoux M. (2001). Fitness and sexual response to population density in Daphnia pulex. Freshwater Biology 46: 667–677
Betancourt A. J. and Presgraves D. C. (2002). Linkage limits the power of natural selection in Drosophila. Proceedings of the National Academy of Sciences USA 99: 13616–13620
Boersma M. and Spaak P. (1999). Environmental stress and local adaptation in Daphnia magna. Limnology and Oceanography 44: 393–402
Boersma M. and Spaak P. (1998). Predator-mediated plasticity in morphology, life history and behavior of Daphnia: the uncoupling of responses. American Naturalist 152: 237–248
Brewer M. C. (1998). Mating behaviours of Daphnia pulicaria, a cyclic parthenogen: comparisons with copepods. Philosophical Transactions of the Royal Society of London B 353: 805–815
Burns C. W. (1995). Effects of crowding and different food levels on growth and reproductive investment of Daphnia. Oecologia 101: 234–244
Cáceres C. E. and Tessier A. J. (2004). Incidence of diapause varies among populations of Daphnia pulicaria. Oecologia 141: 425–431
Carmona M. J., Serra M. and Miracle M. R. (1993). Relationships between mixis in Brachionus plicatilis and preconditioning of culture medium by crowding. Hydrobiologia 255/256: 145–152
Carvalho A. B., Sampaio M. C., Varandas F. R. and Klaczko L. B. (1998). An experimental demonstration of Fisher’s Principle: evolution of sexual proportion by natural selection. Genetics 148: 719–731
Carvalho G. R. and Hughes R. N. (1983). The effect of food availability, female culture-density and photoperiod on ephippia production in Daphnia magna Straus (Crustacea: Cladocera). Freshwater Biology 13: 37–46
Chadwick W. and Little T. J. (2005). A parasite-mediated life-history shift in Daphnia magna. Proceedings of the Royal Society of London B 272: 505–509
Cleuvers M., Goser B. and Ratte H.-T. (1997). Life-history shift by intraspecific interaction in Daphnia magna: change in reproduction from quantity to quality. Oecologia 110: 337–345
Crease T. J. and Hebert P. D. N. (1983). A test for the production of sexual pheromones by Daphnia magna (Crustacea: Cladocera). Freshwater Biology 13: 491–496
Declerck S. (2005). The study of biodiversity in freshwater habitats: societal relevance and suggestions for priorities in science policy. Hydrobiologia 542: 1–9
Vanoverbeke J. (1999). An uncoupling of male and sexual egg production leads to reduced inbreeding in the cyclical parthenogen Daphnia. Proceedings of the Royal Society of London B 266: 2471–2477
Deng H.-W. (1996). Environmental and genetic control of sexual reproduction in Daphnia. Heredity 76: 449–458
Deng H.-W. (1997a). Increase in developmental instability upon inbreeding in Daphnia. Heredity 78: 182–189
Deng H.-W. (1997b). Photoperiodic response of sexual reproduction in the Daphnia pulex group is reversed in two distinct habitats. Limnology and Oceanography 42: 609–611
Dudycha J. L. (2004). Mortality dynamics of Daphnia in contrasting habitats and their role in ecological divergence. Freshwater Biology 49: 505–514
(2000). Biological Test Method: Reference Method for Determining Acute Lethality of Effluents to Daphnia magna. Environmental Technology Centre, Ottawa
Ferrari D. C. and Hebert P. D. N. (1982). The induction of sexual reproduction in Daphnia magna: genetic differences between arctic and temperate populations. Canadian Journal of Zoology 60: 2143–2148
Fisher R. A. (1930). The Genetical Theory of Natural Selection. Claredon Press, Oxford
Fitzsimmons J. M. and Innes D. J. (2005). No evidence of Wolbachia among Great Lakes area populations of Daphnia pulex (Crustacea: Cladocera). Journal of Plankton Research 27: 121–124
Glazier D. S. (1992). Effects of food, genotype and maternal size and age on offspring investment in Daphnia magna. Ecology 73: 910–926
Gliwicz Z. M. and Guisande C. (1992). Family planning in Daphnia: resistance to starvation in offspring born to mothers grown at different food levels. Oecologia 91: 463–467
Gustafsson S., Rengefors K. and Hansson L.-A. (2005). Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 86: 2561–2567
Hadany L. and Feldman M. W. (2005). Evolutionary traction: the cost of adaptation and the evolution of sex. Journal of Evolutionary Biology 18: 309–314
Hebert P. D. N. (1987). Genotypic characteristics of the Cladocera. Hydrobiologia 145: 183–193
Hebert P. D. N., Beaton M. J., Schwartz S. S. and Stanton D. J. (1989). Polyphyletic origins of asexuality in Daphnia pulex. I. breeding-system variation and levels of clonal diversity. Evolution 43: 1004–1015
Hebert P. D. N. and Crease T. (1983). Clonal diversity in populations of Daphnia pulex reproducing by obligate parthenogenesis. Heredity 51: 353–369
Hobæk A. and Larsson P. (1990). Sex determination in Daphnia magna. Ecology 71: 2255–2268
Hurlbert S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54: 187–211
Innes D. J. (1989). Genetics of Daphnia obtusa: genetic load and linkage analysis in a cyclical parthenogen. Journal of Heredity 80: 6–10
Innes D. J. (1997). Sexual reproduction of Daphnia pulex in a temporary habitat. Oecologia 111: 53–60
Innes D. J. and Dunbrack R. L. (1993). Sex allocation variation in␣Daphnia pulex. Journal of Evolutionary Biology 6: 559–575
Innes D. J., Fox C. J. and Winsor G. L. (2000). Avoiding the cost of males in obligately asexual Daphnia pulex (Leydig). Proceedings of the Royal Society of London B 267: 991–997
Innes D. J. and Hebert P. D. N. (1988). The origin and genetic basis of obligate parthenogenesis in Daphnia pulex. Evolution 42: 1024–1035
Innes D. J., Schwartz S. S. and Hebert P. D. N. (1986). Genotypic diversity and variation in mode of reproduction among populations in the Daphnia pulex group. Heredity 57: 345–355
Innes D. J. and Singleton D. R. (1994). Variation in reproduction and sex allocation among clones of Daphnia pulex. In: Beaumont, A. R. (eds) Genetics and Evolution of Aquatic Organisms, pp 335–342. Chapman and Hall, London
Innes D. J. and Singleton D. R. (2000). Variation in allocation to sexual and asexual reproduction among clones of cyclically parthenogenetic Daphnia pulex (Crustacea: Cladocera). Biological Journal of the Linnean Society 71: 771–787
Kerfoot W. C. and Weider L. J. (2004). Experimental paleoecology (resurrection ecology): chasing Van Valen’s Red Queen hypothesis. Limnology and Oceanography 49: 1300–1316
Kessler K. and Lampert W. (2004). Fitness optimization of Daphnia in a trade-off between food and temperature. Oecologia 140: 381–387
Kleiven O. T., Larsson P. and Hobæk A. (1992). Sexual reproduction in Daphnia magna requires three stimuli. Oikos 65: 197–206
Korpelainen H. (1986). The effects of temperature and photoperiod on life history parameters of Daphnia magna (Crustacea: Cladocera). Freshwater Biology 16: 615–620
Korpelainen H. (1992). Lowered female reproductive effort as an indicator for increased male production and sexuality in Daphnia (Crustacea: Cladocera). Invertebrate Reproduction and Development 22: 281–290
LaMontagne J. M. and McCauley E. (2001). Maternal effects in Daphnia: what mothers are telling their offspring and do they listen?. Ecology Letters 4: 64–71
Larsson P. (1991). Intraspecific variability in response to stimuli for male and ephippia formation in Daphnia pulex. Hydrobiologia 225: 281–290
Lass S. and Bittner K. (2002). Facing multiple enemies: parasitised hosts respond to predator kairomones. Oecologia 132: 344–349
Loaring J. M. and Hebert P. D. N. (1981). Ecological differences among clones of Daphnia pulex Leydig. Oecologia 51: 162–168
López S. and Domínguez C. A. (2003). Sex choice in plants: facultative adjustment of the sex ratio in the perennial herb Begonia gracilis. Journal of Evolutionary Biology 16: 1177–1185
Lürling M., Roozen F. and Goser B. (2003). Response of Daphnia to substances released from crowded congeners and conspecifics. Journal of Plankton Research 25: 967–978
Lushai G., Loxdale H. D. and Allen J. A. (2003). The dynamic clonal genome and its adaptive potential. Biological Journal of the Linnean Society 79: 193–208
Lynch M., Spitze K. and Crease T. (1989). The distribution of life-history variation in the Daphnia pulex complex. Evolution 43: 1724–1736
Lynch M., Weider L. J. and Lampert W. (1986). Measurement of the carbon balance in Daphnia. Limnology and Oceanography 31: 17–33
Mikulski A., Czernik M. and Pijanowska J. (2005). Induction time and reversibility of changes in Daphnia life history caused by the presence of fish. Journal of Plankton Research 27: 757–762
Mitchell S. E. and Read A. F. (2005). Poor maternal environment enhances offspring disease resistance in an invertebrate. Proceedings of the Royal Society of London B 272: 2601–2607
Mitchell S. E., Read A. F. and Little T. J. (2004). The effect of a pathogen epidemic on the genetic structure and reproductive strategy of the crustacean Daphnia magna. Ecology Letters 7: 848–858
Mitchell S. E., Rogers E. S., Little T. J. and Read A. F. (2005). Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59: 70–80
Mu X. and LeBlanc G. A. (2002). Environmental antiecdysteroids alter embryo development in the crustacean Daphnia magna. Journal of Experimental Zoology 292: 287–292
Nelson W. A., McCauley E. and Wrona F. J. (2005). Stage-structured cycles promote genetic diversity in a predator-prey system of Daphnia and algae. Nature 433: 413–417
(1998). OECD Guidelines for Testing of Chemicals: Daphnia magna Reproduction Test. Organisation for Economic Co-operation and Development, Paris
Olmstead A. W. and LeBlanc G. A. (2001). Temporal and quantitative changes in sexual reproductive cycling of the cladoceran Daphnia magna by a juvenile hormone analog. Journal of Experimental Zoology 290: 148–155
Olmstead A. W. and LeBlanc G. A. (2002). Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. Journal of Experimental Zoology 293: 736–739
Paland S., Colbourne J. K. and Lynch M. (2005). Evolutionary history of contagious asexuality in Daphnia pulex. Evolution 59: 800–813
Peer K. and Taborsky M. (2004). Female ambrosia beetles adjust their offspring sex ratio according to outbreeding opportunities for their sons. Journal of Evolutionary Biology 17: 257–264
Pijanowska J. and Kowalczewski A. (1997). Cues from injured Daphnia and from cyclopoids feeding on Daphnia can modify life histories of conspecifics. Hydrobiologia 350: 99–103
Printes L. B. and Callaghan A. (2003). Intraclonal variability in Daphnia acetylcholinesterase activity: the implications for its applicability as a biomarker. Environmental Toxicology and Chemistry 22: 2042–2047
Rice W. R. and Chippindale A. K. (2001). Sexual recombination and the power of selection. Science 294: 555–559
Ruvinsky A. O., Perelygin A. A., Lobkov Y. I. and Belyaev D. K. (1986). Factors organising and maintaining polymorphism in a cyclic parthenogenetic species: Daphnia pulex. Heredity 57: 15–22
Salathé P. and Ebert D. (2003). The effects of parasitism and inbreeding on the competitive ability in Daphnia magna: evidence for synergistic epistasis. Journal of Evolutionary Biology 16: 976–985
Sanders R. W., Williamson C. E., Stutzman P. L., Moeller R. E., Goulden C. E. and Aoki-Goldsmith R. (1996). Reproductive success of “herbivorous” zooplankton fed algal and nonalgal food resources. Limnology and Oceanography 41: 1295–1305
Sarre S. D., Georges A. and Quinn A. (2004). The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. BioEssays 26: 639–645
Serra M., Snell T. W. and Gilbert J. J. (2005). Delayed mixis in rotifers: an adaptive response to the effects of density-dependent sex on population growth. Journal of Plankton Research 27: 37–45
Simon J.-C., Delmotte F., Rispe C. and Crease T. (2003). Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biological Journal of the Linnean Society 79: 151–163
Simon J.-C., Rispe C. and Sunnucks P. (2002). Ecology and evolution of sex in aphids. Trends in Ecology and Evolution 17: 34–39
Ślusarczyk M. (1995). Predator-induced diapause in Daphnia. Ecology 76: 1008–1013
Spaak P., Denk A., Boersma M. and Weider L. J. (2004). Spatial and temporal patterns of sexual reproduction in a hybrid Daphnia species complex. Journal of Plankton Research 26: 625–635
Stabell O. B., Ogbebo F. and Primicerio R. (2003). Inducible defences in Daphnia depend on latent alarm signals from conspecific prey activated in predators. Chemical Senses 28: 141–153
Stross R. G. (1969). Photoperiod control of diapause in Daphnia. III. Two-stimulus control of long-day, short-day induction. Biological Bulletin 137: 359–374
Tessier A. J. and Cáceres C. E. (2004). Differentiation in sex investment by clones and populations of Daphnia. Ecology Letters 7: 695–703
Trivers R. L. and Willard D. E. (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science 179: 90–92
(2002). Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. U.S. Environmental Protection Agency, Washington
Valenzuela N., Adams D. C. and Janzen F. J. (2003). Pattern does not equal process: exactly when is sex environmentally determined?. American Naturalist 161: 676–683
Vrijenhoek R. C. (1998). Animal clones and diversity: are natural clones generalists or specialists?. BioScience 48: 617–628
Weber A. and Declerck S. (1997). Phenotypic plasticity of Daphnia life history traits in response to predator kairomones: genetic variability and evolutionary potential. Hydrobiologia 360: 89–99
Weetman D. and Atkinson D. (2002). Antipredator reaction norms for life history traits in Daphnia pulex: dependence on temperature and food. Oikos 98: 299–307
Weinzierl R. P., Schmidt P. and Michiels N. K. (1999). High fecundity and low fertility in parthenogenetic planarians. Invertebrate Biology 118: 87–94
Whittingham L. A., Dunn P. O. and Nooker J. K. (2005). Maternal influences on brood sex ratios: an experimental study in tree swallows. Proceedings of the Royal Society of London B 272: 1775–1780
Winsor G. L. and Innes D. J. (2002). Sexual reproduction in Daphnia pulex (Crustacea: Cladocera): observations on male mating behaviour and avoidance of inbreeding. Freshwater Biology 47: 441–450
Yampolsky L. Y. (1992). Genetic variation in the sexual reproduction rate within a population of a cyclic parthenogen, Daphnia magna. Evolution 46: 833–837
Yasumoto K., Nishigami A., Yasumoto M., Kasai F., Okada Y., Kusumi T. and Ooi T. (2005). Aliphatic sulfates released from Daphnia induce morphological defense of phytoplankton: isolation and synthesis of kairomones. Tetrahedron Letters 46: 4765–4767
Zhang L. and Baer K. N. (2000). The influence of feeding, photoperiod and selected solvents on the reproductive strategies of the water flea, Daphnia magna. Environmental Pollution 110: 425–430
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fitzsimmons, J.M., Innes, D.J. Inter-genotype variation in reproductive response to crowding among Daphnia pulex . Hydrobiologia 568, 187–205 (2006). https://doi.org/10.1007/s10750-006-0104-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0104-5