Abstract
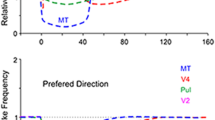
This paper is about how cortical recurrent interactions in primary visual cortex (V1) together with feedback from extrastriate cortex can account for spectral peaks in the V1 local field potential (LFP). Recent studies showed that visual stimulation enhances the γ-band (25–90 Hz) of the LFP power spectrum in macaque V1. The height and location of the γ-band peak in the LFP spectrum were correlated with visual stimulus size. Extensive spatial summation, possibly mediated by feedback connections from extrastriate cortex and long-range horizontal connections in V1, must play a crucial role in the size dependence of the LFP. To analyze stimulus-effects on the LFP of V1 cortex, we propose a network model for the visual cortex that includes two populations of V1 neurons, excitatory and inhibitory, and also includes feedback to V1 from extrastriate cortex. The neural network model for V1 was a resonant system. The model’s resonance frequency (ResF) was in the γ-band and varied up or down in frequency depending on cortical feedback. The model’s ResF shifted downward with stimulus size, as in the real cortex, because increased size recruited more activity in extrastriate cortex and V1 thereby causing stronger feedback. The model needed to have strong local recurrent inhibition within V1 to obtain ResFs that agree with cortical data. Network resonance as a consequence of recurrent excitation and inhibition appears to be a likely explanation for γ-band peaks in the LFP power spectrum of the primary visual cortex.










Similar content being viewed by others
References
Angelucci, A., Levitt, J. B., Walton, E. J., Hupe, J. M., Bullier, J., & Lund, J. S. (2002). Circuits for local and global signal integration in primary visual cortex. Neuroscience, 22, 8633–8646.
Belitski, A., Gretton, A., Magri, C., Murayama, Y., Montemurro, M. A., Logothetis, N. K., et al. (2008). Low-Frequency Local Field Potentials and Spikes in Promary Visul Cortex Convey Independent Visual Information. Journal of Neuroscience, 28, 5696–709.
Ben-Yishai, R., Bar-Or, R., & Sompolinsky, H. (1995). Theory of orientation tuning in visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 92, 3844–3848.
Borg-Graham, L. J., Monier, C., Frégnac, Y. (1998). Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature, 393(6683), 369–73.
Borgers, C., & Kopell, N. J. (2008). Gamma oscillation and stimulus selection. Neural Computation, 20, 383–414.
Brunel, N., & Wang, X. J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. Journal of Neurophysiology, 90, 415–430.
Burns, S. P., Shapley, R. M., Shelley, M. J., & Xing, D. (2008). Searching for phase coherence in the cortical network with time-frequency analysis of the local field potential. Society for Neuroscience Abstracts, 38, 163.4.
Buzsaki, G. (2006). Rhythms of the brain. New York: Oxford UP.
Cardin, J. A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., et al. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459(7247), 663–7.
Eckhorn, R., Bauer, R., Jordan, W., Brosch, M., Kruse, W., Munk, M., et al. (1988). Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biological Cybernetics, 60, 121–130.
Eckhorn, R., Frien, A., Bauer, R., Woelbern, T., Kehr, H. (1993). High frequency (60–90 Hz) oscillations in primary visual cortex of awake monkey. Neuroreport, 4(3), 243–6.
Freeman, W. J. (1975). Mass action in the nervous system. New York: Academic.
Gieselmann, M. A., & Thiele, A. (2008). Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. European Journal of Neuroscience, 28, 447–459.
Goldberg, J. A., Rokni, U., & Sompolinsky, H. (2004). Patterns of Ongoing Activity and the Functional Architectureof the Primary Visual Cortex. Neuron, 42, 489–500.
Gonchar, Y., & Burkhalter, A. (2003). Distinct GABAergic targets of feedforward and feedback connections between lower and higher areas of rat visual cortex. Journal of Neuroscience, 23, 10904–12.
Gray, C. M., Konig, P., Engle, A. K., & Singer, W. (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature, 338, 334–337.
Hasenstaub, A., Shu, Y., Haider, B., Kraushaar, U., Duque, A., & McCormick, D. A. (2005). Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron, 47(3), 423–35.
Henrie, J. A., & Shapley, R. (2005). LFP power spectra in V1 cortex: the graded effect of stimulus contrast. Journal of Neurophysiology, 94(1), 479–90.
Henrie, J. A., Kang, K., & Shapley, R. (2005). Stimulus size affects the LFP spectral contents in primate V1. Society for Neuroscience Abstracts, 35, 284.18.
Hirsch, J. A., Alonso, J. M., Reid, R. C., & Martinez, L. M. (1998). Synaptic integration in striate cortical simple cells. Journal of Neuroscience, 18, 9517–28.
Ishikane, H., Gangi, M., Honda, S., & Tachibana, M. (2005). Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nature Neuroscience, 8(8), 1087–95.
Kang, K., Shelley, M. J., & Sompolinsky, H. (2003). Mexican hats and pinwheels in visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 100(5), 2848–2853.
Kruse, W., & Eckhorn, R. (1996). Inhibition of sustained gamma oscillations (35–80 Hz) by fast transient responses in cat visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 93, 6112–6117.
Leung, L. S. (1982). Nonlinear feedback model of neuronal popultions in hippocampal CA1 region. Journal of Neuophysiology, 47, 845–868.
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–7.
Lund, J. S. (1988). Anatomical organization of macaque monkey striate visual cortex. Annual Reviews of Neuroscience, 11, 253–88.
Lund, J. S., Angelucci, A., & Bressloff, P. C. (2003). Anatomical substrates for functional columns in macaque monkey primary visual cortex. Cerebral Cortex, 13, 15–24.
Mazzoni, A., Panzeri, S., Logothetis, N. K., & Brunel, N. (2008). Encoding of Naturalistic Stimuli by Local Field Potential Spectra in Networks of Excitatory and Inhibitory Neurons. PLOS Computational Biology, 4, e10000239.
McCormick, D. A., Shu, Y., Hasenstaub, A., Sanchez-Vives, M., Badoual, M., & Bal, T. (2003). Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cerebral Cortex, 13(11), 1219–31.
McLaughlin, D., Shapley, R., Shelley, M., & Wielaard, J. (2000). A neuronal network model of macaque primary visual cortex (V1): orientation selectivity and dynamics in the input layer 4Calpha. Proceedings of the National Academy of Sciences of the United States of America, 97, 8087–8092.
Montgomery, S. M., & Buzsáki, G. (2007). Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proceedings of the National Academy of Sciences of the United States of America, 104(36), 14495–500.
Penttonen, M., Kamondi, A., Acsady, L., & Buzsaki, G. (1998). Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. European Journal of Neuroscience, 10(2), 718–28.
Priebe, N. J., & Ferster, D. (2008). Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron, 57, 482–97.
Rennie, C. J., Wright, J. J., & Robinson, P. A. (2000). Mechanisms of Cortical Electrical Activity and Emergence of Gamma Rhythm. Journsl of Theoretical Biology, 205, 17–35.
Ringach, D. L., Hawken, M. J., & Shapley, R. (1997). Dynamics of orientation tuning in macaque primary visual cortex. Nature (London), 387, 281–284.
Robinson, P. A. (2006). Patch propagators, brain dynamics, and the generation of spatially structured gamma oscillation. Physical Review E, 73, 041904.
Schwabe, L., Obermayer, K., Angelucci, A., & Bressloff, P. C. (2006). The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model. Journal of Neuroscience, 26(36), 9117–29.
Shelley, M., & McLaughlin, D. (2002). Coarse-grained reduction and analysis of a network model of cortical response: I. Drifting grating stimuli. Journal of Computer Neuroscience, 12(2), 97–122.
Shriki, O., Hansel, D., & Sompolinsky, H. (2003). Rate models for conductance-based cortical neuronal networks. Neural Computation, 15(8), 1809–41.
Somers, D., Nelson, S., & Sur, M. (1995). An emergent model of orientation selectivity in cat visual cortical simple cells. Journal of Neuroscience, 15, 5448–5465.
Steriade, M. (2001). Intact and Sliced Brain. Cambridge: MIT.
Steriade, M., Amzica, F., & Contreras, D. (1996). Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. Journsl of Neuroscience, 16, 392–417.
Tao, L., Cai, D., McLaughlin, D. W., Shelley, M. J., & Shapley, R. (2006). Orientation selectivity in visual cortex by fluctuation-controlled criticality. Proceedings of the National Academy of Sciences of the United States of America, 103(34), 12911–6.
Traub, R., Whittington, M. A., Stanford, I. M., & Jefferys, J. G. R. (1996). Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. Journal of Neuroscience, 21, 9478–9486.
Troyer, T., Krukowski, A., Priebe, N., & Miller, K. (1998). Contrast-invariant orientation tuning in cat visual cortex: thalamocortical input tuning and correlation-based intracortical connectivity. Journal of Neuroscience, 18, 5908–5927.
Volgushev, M., Pei, X., Vidyasagar, T. R., & Creutzfeldt, O. D. (1993). Excitation and inhibition in orientation selectivity of cat visual cortex neurons revealed by whole-cell recordings in vivo. Visual Neuroscience, 10, 1151–5.
Weisstein, E. W. Routh-Hurwitz Theorem. From MathWorld—A Wolfram Web Resource. http://mathworld.wolfram.com/Routh-HurwitzTheorem.html.
Womelsdorf, T., Schoffelen, J. M., Oostenveld, R., Singer, W., Desimone, R., Engel, A. K., et al. (2007). Modulation of neuronal interactions through neuronal synchronization. Science, 316, 1609–12.
Zeitler, M., Fries, P., Gielen, S. (2006). Assessing neuronal coherence with single-unit, multi-unit, and local field potentials. Neural Computation, 18(9), 2256–81.
Acknowledgements
This work was supported by the Swartz Foundation, grant EY01472 of National Institute of Health and grant 0745253 of National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
1.1 Rate equation and time course of conductance change
Consider the time evolution of conductance for AMPA receptors in a neuron. This can be described by an integral. \( m(t) = \int {A_E \left( {t - a} \right)f_E (a)da} \). f E (t) is \( \sum\nolimits_j {\delta \left( {a - t_j^i } \right)} \) where \( t_j^i \) is ith spikes from jth presynaptic excitatory neurons and δ(t) is a delta function. A E (t) is the time course of conductance change of AMPA receptors. The conductance due to GABAA receptors, n(t) can be described with another function, A I (t) in the similar way.
When the number of presynaptic neurons is large and the neural network is in an asynchronous state, f E (t) can be replaced by the mean firing rate of presynaptic neurons, \( \overline{f}_E (t) \) and the mean firing rates are determined by the conductances of the presynaptic neurons: \( \bar{f}_E (t) = \bar{f}_E \left( {m(t),n(t),I(t)} \right) \) where I(t) is the feed-forward input to the network. Self-consistency requires that m(t) and n(t) should satisfy the following integral equations.
Once we have the solution of the above integral equations, the firing rate of neurons is given by the time derivative of m(t) and n(t). But an integral equation is difficult to analyze and we simplify the integral equations with two approximations. First, we transform the integral equations into differential equations assuming that explicit forms of A E (t) and A I (t) are given. For example, for an exponential model, \( A_E (t) = \exp \left( { - t/\tau_E } \right)/\tau_E \),
where τ E is the decay time constant of the conductance. For AMPA, τ E is a few milliseconds. while for NMDA, τ E is 50–100 msec.
For the difference of exponential model (DOE) with time delay, \( A_E (t) = \left( {e^{{ - (t - \delta )/\tau_E }} - e^{{ - (t - \delta )/\tau_{{E0}} }} } \right)/(\tau_E - \tau_{{E0}} ) \), we find
where δ is the synaptic delay time, and τE0 is the rising time constant of the conductance’s time course. For small τE0, Eq. A.4 and A.5 can be merged into one equation.
Another approximation that achieves the same result as Eq. 1.0 is to assume an explicit form of the mean firing rate, \( \bar{f}_E (t) = \bar{f}_E \left( {m(t),n(t),I(t)} \right) \). The linear form with rectifying nonlinearity is known to be a good approximation for conductance based model neurons (Shriki et al. 2003). When the firing rate fluctuates with small amplitude, a series expansion of firing rate in terms of synaptic conductance variables gives us a linear form as well (Brunel and Wang 2003). See also Shelley and McLaughlin (2002) for a coarse-grained reduction of an integrate-and-fire, conductance-based neural network model to a rate model.
Appendix B
2.1 Eigenvalue and ResF with feedback from extrastriate cortex
The characteristic equation of the matrix A in Eq. 3.3 is
The conditions for Eq. B.1 to have eigenvalues with positive real part can be obtained by the Routh-Hurwitz theorem (Weisstein). But here we use a different derivation showing the stability conditions in Eq. 4.2–4 explicitly. The three eigenvalues are either three real numbers or one real number and two complex conjugates. In any case, TrA > 0 if the real parts of all eigenvalues are positive. When one of the real eigenvalues changes in sign from positive to negative, DetA is 0 because DetA is the product all eigenvalues. It means DetA > 0. Finally, when the real parts of two complex eigenvalues change their sign, the three eigenvalues have the form ε ± αi and β where ε,α and β are three real numbers. It is easy to prove the following using the relation between roots and coefficients of the equation.
Therefore, the conditions for the real parts of all eigenvalues to be positive are given in the following way.
where \( T = \tau_E^{{ - 1}} \left( {1 - S_{{EE}} } \right) + \tau_I^{{ - 1}} \left( {1 + S_{{II}} } \right) \) and \( D = \left( {1 - S_{{EE}} } \right)\left( {1 + S_{{II}} } \right) + S_{{EI}} S_{{IE}} . \)
When TrA * R = DetA, the real part of the complex eigenvalues are zero and \( R = DetA/TrA = \left( {2\pi \nu } \right)^2 . \)
Since is proportional to the inverse of the real part of the eigenvalue, τ damp = ∞ on the stability line defined by the Eq. B.11. Equation B.6 shows that \( R = DetA/TrA = \left( {2\pi \nu } \right)^2 \) in this case. Then the ResF, v obeys
Rights and permissions
About this article
Cite this article
Kang, K., Shelley, M., Henrie, J.A. et al. LFP spectral peaks in V1 cortex: network resonance and cortico-cortical feedback. J Comput Neurosci 29, 495–507 (2010). https://doi.org/10.1007/s10827-009-0190-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0190-2