Abstract
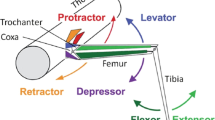
The biomechanical conditions for walking in the stick insect require a modeling approach that is based on the control of pairs of antagonistic motoneuron (MN) pools for each leg joint by independent central pattern generators (CPGs). Each CPG controls a pair of antagonistic MN pools. Furthermore, specific sensory feedback signals play an important role in the control of single leg movement and in the generation of inter-leg coordination or the interplay between both tasks. Currently, however, no mathematical model exists that provides a theoretical approach to understanding the generation of coordinated locomotion in such a multi-legged locomotor system. In the present study, I created such a theoretical model for the stick insect walking system, which describes the MN activity of a single forward stepping middle leg and helps to explain the neuronal mechanisms underlying coordinating information transfer between ipsilateral legs. In this model, CPGs that belong to the same leg, as well as those belonging to different legs, are connected by specific sensory feedback pathways that convey information about movements and forces generated during locomotion. The model emphasizes the importance of sensory feedback, which is used by the central nervous system to enhance weak excitatory and inhibitory synaptic connections from front to rear between the three thorax-coxa-joint CPGs. Thereby the sensory feedback activates caudal pattern generation networks and helps to coordinate leg movements by generating in-phase and out-of-phase thoracic MN activity.
















Similar content being viewed by others
References
Akay, T., & Büschges, A. (2006). Load signals assist the generation of movement dependent reflex reversal in the femur-tibia joint of stick insects. Journal of Neurophysiology, 96, 3532–3537.
Akay, T., Bässler, U., & Büschges, A. (2001). The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. Journal of Neurophysiology, 85, 594–604.
Akay, T., Haehn, S., Schmitz, J., & Büschges, A. (2004). Signals from load sensors underlie interjoint coordination during stepping movements of the stick insect leg. Journal of Neurophysiology, 92, 42–51.
Akay, T., McVea, D. A., Tachibana, A., & Pearson, K. G. (2006). Coordination of fore and hind leg stepping in cats on a transversely-split treadmill. Experimental Brain Research, 175, 211–222.
Akay, T., Ludwar, B. C., Goeritz, M., Schmitz, J., & Büschges, A. (2007). Segment specificity of load signal processing depends on walking direction in the stick insect leg muscle control system. Journal of Neuroscience, 27, 3285–3294.
Bässler, U. (1977). Sense organs in the femur of the stick insect and their relevance to the control of position of the femur-tibia-joint. Journal of Comparative Physiology A, 121, 99–113.
Bässler, U. (1986). Afferent control of walking movements in the stick insect Cuniculina impigra. Journal of Comparative Physiology A, 158, 345–349.
Bässler, U. (1988). Functional principles of pattern generation for walking movements of stick insect forelegs: the role of the femoral chordotonal organ afferences. Journal of Experimental Biology, 136, 125–147.
Bässler, U., & Büschges, A. (1998). Pattern generation for stick insect walking movements—multisensory control of a locomotor program. Brain Research Reviews, 27, 65–88.
Borgmann, A., Scharstein, H., & Büschges, A. (2007). Intersegmental coordination: influence of a single walking leg on the neighboring segments in the stick insect walking system. Journal of Neurophysiology, 98, 1685–1696.
Borgmann, A., Hooper, S. L., & Büschges, A. (2009). Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. Journal of Neuroscience, 29, 2972–2983.
Burrows, M. (1996). The neurobiology of an insect brain. Oxford: Oxford University Press.
Büschges, A. (1995). Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. Journal of Neurobiology, 27, 488–512.
Büschges, A. (1998). Inhibitory synaptic drive patterns motoneuronal activity in rhythmic preparations of isolated thoracic ganglia in the stick insect. Brain Research, 783, 262–271.
Büschges, A. (2005). Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. Journal of Neurophysiology, 93, 1127–1135.
Büschges, A., & El Manira, A. (1998). Sensory pathways and their modulation in the control of locomotion. Current Opinion in Neurobiology, 8, 733–739.
Büschges, A., & Gruhn, M. (2008). Mechanosensory feedback in walking: from joint control to locomotor patterns. Advances in Insect Physiology, 34, 193–230.
Büschges, A., & Schmitz, J. (1991). Nonspiking pathways opposing the resistance reflex in the subcoxal joint of stick insects. Journal of Neurobiology, 22, 224–237.
Büschges, A., Kittmann, R., & Schmitz, J. (1994). Identified nonspiking interneurons in leg reflexes and during walking in the stick insect. Journal of Comparative Physiology A, 174, 685–700.
Büschges, A., Schmitz, J., & Bässler, U. (1995). Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. Journal of Experimental Biology, 198, 435–456.
Büschges, A., Ludwar, B. C., Bucher, D., Schmidt, J., & DiCaprio, R. A. (2004). Synaptic drive contributing to rhythmic activation of motoneurons in the deafferented stick insect walking system. European Journal of Neuroscience, 12, 1856–1862.
Büschges, A., Akay, T., Gabriel, J. P., & Schmidt, J. (2008). Organizing network action for locomotion: insights from studying insect walking. Brain Research Review, 57, 162–171.
Calabrese, R. L. (1995). Half-center oscillators underlying rhythmic movements. In M. Arbib (Ed.), The handbook of brain theory and neural networks (pp. 444–447). Cambridge, MA: MIT press.
Cattaert, D., & LeRay, D. (2001). Adaptive motor control in crayfish. Progress in Neurobiology, 63, 199–240.
Cruse, H. (1980). A quantitative model of walking incorporating central and peripheral influences. Biological Cybernetics, 37, 136.
Cruse, H. (1985a). Which parameters control the leg movement of a walking insect? I. Velocity control during the stance phase. Journal of Experimental Biology, 116, 343–355.
Cruse, H. (1985b). Which parameters control the leg movement of a walking insect? II. The start of the swing phase. Journal of Experimental Biology, 116, 357–362.
Cruse, H. (1990). What mechanisms coordinate leg movement in walking arthropods? Trends in Neuroscience, 13, 15–21.
Cruse, H., & Müller, U. (1986). Two coupling mechanisms which determine the coordination of ipsilateral legs in the walking crayfish. Journal of Experimental Biology, 121, 349–369.
Cruse, H., Kindermann, T., Schumm, M., Dean, J., & Schmitz, J. (1998). Walknet—a biologically inspired network to control six-legged walking. Neural Networks, 1, 1435–1447.
Cruse, H., et al. (2000). A simple neural network for the control of a six-legged walking system. In P. Crago & J. Winters (Eds.), Biomechanics and neural control of posture and movement (pp. 231–239). New York: Springer.
Daun, S., Rybak, I. A., & Rubin, J. (2009). The response of a half-center oscillator to external drive depends on the intrinsic dynamics of its components: a mechanistic analysis. Journal of Computational Neuroscience, 27, 3–36.
Dean, J., & Wendler, G. (1984). Stick insect locomotion on a wheel: patterns of stopping and starting. Journal of Experimental Biology, 110, 203–216.
Delcomyn, F. (1971). The locomotion of the cockroach Periplaneta Americana. Journal of Experimental Biology, 54, 443–452.
Delcomyn, F. (1989). Walking in the American cockroach: the timing of motor activity in the legs during straight walking. Biological Cybernetics, 60, 373–384.
Driesang, R. B., & Büschges, A. (1993). The neural basis of catalepsy in the stick insect. IV. Properties of nonspiking interneurons. Journal of Comparative Physiology A, 173, 445–454.
Dürr, V., Schmitz, J., & Cruse, H. (2004). Behavior-based modelling of hexapod locomotion: linking biology and technical application. Arthropod Structure & Development, 33, 1–13.
Duysens, J., Clarac, F., & Cruse, H. (2000). Load-regulating mechanisms in gait and posture: comparative aspects. Physiological Reviews, 80, 83–133.
Ekeberg, Ö., & Pearson, K. G. (2005). Computer simulation of stepping in the hind legs of the cat: an examination of the mechanisms regulating the stance-to-swing transition. Journal of Neurophysiology, 94, 4256–4268.
Ekeberg, Ö., Blümel, M., & Büschges, A. (2004). Dynamic simulation of stick insect walking. Arthropod Structure & Development, 33, 287–300.
Fischer, H., Schmidt, J., & Büschges, A. (2001). Pattern generation for walking and searching movements of a stick insect leg I. Coordination of motor activity. Journal of Neurophysiology, 85, 341–353.
Foth, E., & Bässler, U. (1985a). Leg movements of stick insects walking with five legs on a treadwheel and with one leg on a motor-driven belt. I. General results and 1:1-coordination. Biological Cybernetics, 51, 313–318.
Foth, E., & Bässler, U. (1985b). Leg movements of stick insects walking with five legs on a treadwheel and with one leg on a motor-driven belt. II. Leg Coordination when step-frequencies differ from leg to leg. Biological Cybernetics, 51, 319–324.
Gal, R., & Libersat, F. (2006). New vistas on the initiation and maintenance of insect motor behaviors revealed by specific lesions of the head ganglia. Journal of Comparative Physiology A, 192, 1003–1020.
Graham, D. (1972). A behavioural analysis of the temporal organisation of walking movements in the 1st instar and adult stick insect (Carausius morosus). Journal of Comparative Physiology, 81, 23–52.
Graham, D. (1977). Simulation of a model for the coordination of leg movement in free walking insects. Biological Cybernetics, 26, 187–198.
Graham, D. (1985). Pattern and control of walking in insects. Advances in Insect Physiology, 18, 31–140.
Grillner, S. (1981). Control of locomotion in bipeds, tetrapods and fish. In V. B. Brooks (Ed.), Handbook of physiology, Sect 1: The Nervous System vol. II: motor control (pp. 1179–1236). Maryland: Waverly Press.
Grillner, S. (2003). The motor infrastructure: from ion channels to neuronal networks. Nature Reviews, 4, 573–586.
Grillner, S., Markram, H., De Schutter, E., Silberberg, G., & LeBeau, F. E. N. (2005). Microcircuits in action—from CPGs to neocortex. Trends in Neurosciences, 28, 525–533.
Gruhn, M., von Uckermann, G., Westmark, S., Woznitza, A., Büschges, A., & Borgmann, A. (2009). Control of stepping velocity in the stick insect Carausius morosus. Journal of Neurophysiology, 102, 1180–1192.
Haridas, C., & Zehr, E. P. (2003). Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. Journal of Neurophysiology, 90, 2850–2861.
Hess, D., & Büschges, A. (1999). Role of proprioceptive signals from an insect femur-tibia joint in patterning motoneuronal activity of an adjacent leg joint. Journal of Neurophysiology, 81, 1856–1865.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in a nerve. Journal of Physiology, 117, 500–544.
Holmes, P. J., Full, R. J., Koditschek, D., & Guckenheimer, J. (2006). The dynamics of legged locomtion: models, analysis, and challenges. SIAM Review, 48(2), 207–304.
Ijspeert, A. J. (2008). Central pattern generators for locomotion control in animals and robots: a review. Preprint of Neural Networks, 21, 642–653.
Ijspeert, A. J., Crespi, A., Ryczko, D., & Cabelguen, J. M. (2007). From swimming to walking with a salamander robot driven by a spinal cord model. Science, 315, 1416–1420.
Izhikevich, E. M. (2007). Dynamical systems in neuroscience: The geometry of excitability and bursting. Cambridge: MIT press, Massachusetts Institute of Technology.
Johnston, R. M., & Levine, R. B. (2002). Thoracic leg motoneurons in the isolated CNS of adult Manduca produce patterned activity in response to pilocarpine. Invertebrate Neuroscience, 4, 175–192.
Katz, P. S., & Hooper, S. L. (2007). Invertebrate central pattern generators. In G. North & R. J. Greenspan (Eds.), Invertebrate neurobiology (pp. 251–280). Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Laurent, G., & Burrows, M. (1989a). Distribution of intersegmental inputs to nonspiking local interneurons and motor neurons in the locust. Journal of Neuroscience, 9, 3019–3029.
Laurent, G., & Burrows, M. (1989b). Intersegmental interneurons can control the gain of reflexes in adjacent segments of the locust by their action on nonspiking local interneurons. Journal of Neuroscience, 9, 3030–3039.
Ludwar, B. C., Westmark, S., Büschges, A., & Schmidt, J. (2005). Modulation of membrane potential in mesothoracic moto- and interneurons during stick insect front leg walking. Journal of Neurophysiology, 93, 1255–1265.
Mischenko, E., Kolesov, Y., Kolesov, A., & Rozov, N. (1994). Asymptotic methods in singularly perturbed systems. New York: Consultants Bureau.
Orlovsky, G. N., Deliagina, T. G., & Grillner, S. (1999). Neuronal control of locomotion. Oxford: Oxford Univ Press.
Pearson, K. G. (2004). Generating the walking gait: role of sensory feedback. Progress in Brain Research, 143, 123–129.
Pearson, K., Ekeberg, Ö., & Büschges, A. (2006). Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends in Neurosciences, 29, 625–631.
Prochazka, A. (1996). Proprioceptive feedback and movement regulation. In L. Rowell & J. T. Sheperd (Eds.), Handbook of physiology (pp. 89–127). New York: American Physiological Society.
Roeder, K. D. (1937). The control of tonus and locomotor activity in the praying mantis (Mantis religiosa L.). Journal of Experimental Zoology, 76, 353–374.
Samara, R. F., & Currie, S. N. (2007). Crossed commissural pathways in the spinal hindlimb enlargement are not necessary for right left hindlimb alternation during turtle swimming. Journal of Neurophysiology, 98, 2223–2231.
Satterlie, R. A. (1985). Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science, 229, 402–404.
Schmitz, J. (1986a). Properties of the feedback system controlling the coxa-trochanter joint in the stick insect Carausius morosus. Biological Cybernetics, 55, 35–42.
Schmitz, J. (1986b). The depressor trochanteris motoneurones and their role in the coxa-throchanteral feedback loop in the stick insect Carausius morosus. Biological Cybernetics, 55, 25–34.
Schmitz, J., & Stein, W. (2000). Convergence of load and movement information onto leg motoneurons in insects. Journal of Neurobiology, 43, 424–436.
Selverston, A. I., & Moulins, M. (1985). Oscillatory neural networks. Annual Review Physiology, 47, 29–48.
Skinner, F., Kopell, N., & Marder, E. (1994). Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. Journal of Computational Neuroscience, 1, 69–87.
Sponberg, S., & Full, R. J. (2008). Neuromechanical response of musco-skeletal structures in cockroaches during rapid running on rough terrain. Journal of Experimental Biology, 211, 446.
Stein, W., Büschges, A., & Bässler, U. (2006). Intersegmental information flow in the thoracic nerve cord of the stick insect as revealed by removal of gabaergic inhibition. Journal of Neurobiology, 66, 1253–1269.
Van Drongelen, W., Koch, H., Elsen, F. P., Lee, H. C., Mrejeru, A., Doren, E., et al. (2006). The role of persistent sodium current in bursting activity of mouse neocortical networks in vitro. Journal of Neurophysiology, 96, 2564–2577.
Wendler, G. (1968). Ein Analogmodell der Beinbewegung eines laufenden Insekts. Kybernetik, 18, 67–74.
Wendler, G. (1978). Lokomotion: das Ergebnis zentral-peripherer Interaktion. Verhandlungen der Deutschen Zoologischen Gesellschaft, 80–96.
Westmark, S., Oliveira, E. E., & Schmidt, J. (2009). Pharmacological analysis of tonic activity in motoneurons during stick insect walking. Journal of Neurophysiology, 102, 1049–1061.
Zhong, G., Masino, M. A., & Harris-Warrick, R. M. (2007). Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. Journal of Neuroscience, 27, 4507–4518.
Zill, S. N., Schmitz, J., & Büschges, A. (2004). Leg sensors and sensory-motor interactions. Arthropod Structure & Development, 33, 273–286.
Zill, S. N., Keller, B. R., & Duke, E. R. (2009). Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking. Journal of Neurophysiology, 101, 2297–2304.
Acknowledgments
I like to thank Drs. A. Borgmann, A. Büschges, H. Cruse, M. Gruhn, F. Pasemann, J. Schmidt and T. I. Toth for stimulating discussions in the course of the work.
Grant
This study was supported by Deutsche Forschungsgemeinschaft grant DA1182/1-1 and by generous start-up support from the University of Cologne, Department of Animal Physiology, Cologne, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Frances K. Skinner
Appendices
Appendix
I summarized some central notations used in this paper in the following table:
Interneuron model using the persistent sodium current
The ordinary differential equations for this model are
with associated functions
where
Since I assumed the synaptic coupling to be fast, I set \( s = {s_\infty } \) in the simulations.
The following assumptions are made on system (A1):
(H1) For i ∈ {1,2} and fixed s ∈ I s , the v-nullcline, \( \{ ({v_i},{h_i}):{F_i}({v_i},{h_i},{s_j}) = 0\}, \) defines a cubic shaped curve, composed of left, middle, and right branches, in the (v i ,h i ) phase plane.
Dropping the subscript j from s j , denote the branches of F i = 0 by \( v = {v^i}_L(h,s) \),\( v = {v^i}_M(h,s) \), \( v = {v^i}_R(h,s) \), with \( {v^i}_L < {v^i}_M < {v^i}_R \) for each (h,s) on which all three functions are defined. The variable s corresponds to the synaptic input received by the cell, driven by the voltage of the other cell. The drive current to each cell, \( {g_{app,i}}({v_i} - {v_{app}}) \), is also treated as synaptic but is independent of the other cell in the network.
Specifically, under (H1), for fixed s ∈ I s , the left branch \( ({v_L}(h,s),h) \) meets the middle branch \( ({v_M}(h,s),h) \) in the left knee of the v-nullcline, while the middle branch meets the right branch \( ({v_R}(h,s),h) \) in the right knee. For each s ∈ I s , let \( {p_{LK}}(s) = ({v_{LK}}(s),{h_{LK}}(s)) \) denote the left knee of the v-nullcline and, similarly, let \( {p_{RK}}(s) = ({v_{RK}}(s),{h_{RK}}(s)) \) denote the right knee of the v-nullcline.
The dotted and dashed cubic shaped curves in Fig. 3(a) represent the v-nullclines for s=smax and s=0, respectively.
(H2) For i ∈ {1,2}, the h-nullcline, \( \{ ({v_i},{h_i}):{g_i}({v_i},{h_i}) = 0\} \), is a monotonic curve in the (v i ,h i ) plane. For fixed \( s \in [0,{s_{\max }}[ \), the h-nullcline intersects F i = 0 at a unique point \( {p_{FP}}(s) = ({v_{FP}}(s),{h_{FP}}(s)) \) on the right branch of the corresponding v-nullcline.
A cell is defined as excitable if the intersection point of the h- and v-nullcline p FP (0) lies on the left branch of the v-nullcline, \( \{ (v,h):v = {v_L}(h,0)\} \), as oscillatory if p FP (0) lies on the middle branch of the v-nullcline, \( \{ (v,h):v = {v_M}(h,0)\} \); in this case, the cell will intrinsically oscillate, yielding a reduced representation of bursting activity and finally, a cell is tonic if p FP (0) lies on the right, most depolarized branch of the v-nullcline, \( \{ (v,h):v = {v_R}(h,0)\} \), yielding a reduced representation of tonic spiking, which will be the case in this modeling study. The latter case is illustrated in Fig. 3(a), in which the h-nullcline (solid monotonic curve) intersects each v-nullcline in a unique point on the right branch for s<smax.
One further restriction has to be made on the system (A1):
(H3) For i ∈ {1,2} and s = s max, the h-nullcline intersects F i = 0 either at a unique point p FP,R (s max) on the right branch of the corresponding v-nullcline or at three different points one on the left, one on the middle and one on the right branch of the corresponding v-nullcline, p FP,L (s max), p FP,M (s max), p FP,R (s max), depending on the extra drive g app,i
These two cases are illustrated in Figs. 2(a) and 3(a), respectively.
In a neuron, a bursting solution alternates repeatedly between silent phases of relatively constant, low voltage and active phases featuring voltage spikes, which are rapid voltage oscillations of significant amplitude. A model of the form (A1) can be obtained from a model bursting neuron by omitting spike-generating currents but maintaining a current that allows for transitions to an elevated voltage state. In this model, a bursting solution consists of an oscillation composed of silent phases, with \( {v_i} \approx {v^i}_L(h,s) \), alternating with active phases, with \( {v_i} \approx {v^i}_R(h,s) \).
This situation bears similarities to the activity of non-spiking interneurons in the stick insect central neural networks during rhythmic activity, which are known to participate in rhythm generation in a walking stick insect (Büschges et al. 1994).
2.1 Slow and fast subsystems of the interneuron model
In our previous work (Daun et al. 2009) we established conditions under which periodically oscillating solutions can be constructed for system (A1) under the structural hypotheses (H1), (H2) and (H3) and the parameter choices given above, in the singular limit of ε ↓ 0. Results on geometric singular perturbation theory suggest that this construction will yield the existence of nearby oscillating solutions for ε > 0 sufficiently small (Mischenko et al. 1994).
For system (A1), there are associated fast and slow subsystems. The fast subsystem is obtained by setting ε = 0 directly and thus it takes the form
Recall that i ∈ {1,2}, so Eq. (A2) is a system of six equations.
To define various slow subsystems, set τ = εt and let “dot” denote differentiation with respect to τ. Under this rescaling of time, system (A1) becomes, with i ∈ {1,2}
The slow subsystems are obtained from system (A3) by setting ε = 0, solving the algebraic equations, and inserting the results into the h-equation. This process yields, for each i ∈ {1,2},
for X ∈ {L,M,R}. In Eq. (A4), s j depends on v j and hence is a function of h j .
Consider the limit of σ syn ↓ 0 in the equation \( {s_\infty }(v) = 1/(1 + \exp ((v - {\theta_{syn}})/{\sigma_{syn}})) \). Since the branch v M (h,s) is unstable with respect to the fast subsystem, there are four distinct slow subsystems (A4) that could theoretically be relevant. Two of these are obtained when cell i is silent and cell j active for i = 1 or i = 2, and each of these takes the form
The other two subsystems involve the cases that both cells are silent or active. A key point is that the singular solution consists of a concatenation of solutions of systems (A2) and (A5)–(A6) for i = 1,2. Projected to each (v,h)-plane, the solutions to system (A2) consist of jumps between branches of v-nullclines for different values of s, while the solutions to the slow subsystems take the form of pieces of these nullclines. Although the slow subsystems above correspond to σ syn ↓ 0, solutions obtained in this limit persist for small \( |{\sigma_{syn}}| > 0 \) for the persistent sodium model.
Simplified motoneuron model
The ordinary differential equation of this 1-dimensional model is given by
with associated functions and parameters as above except
The coupling between the interneurons forming the half-center CPG and the motoneurons as well as the intra- and inter-leg couplings are as described in the main text.
Rights and permissions
About this article
Cite this article
Daun-Gruhn, S. A mathematical modeling study of inter-segmental coordination during stick insect walking. J Comput Neurosci 30, 255–278 (2011). https://doi.org/10.1007/s10827-010-0254-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0254-3