Abstract
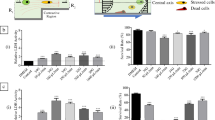
Recent studies have demonstrated that combining cells with meshes prior to implantation successfully enhanced hernia repair. The idea is to create a biologic coating surrounding the mesh with autologous cells, before transplantation into the patient. However, due to the lack of a prompt and robust cell adhesion to the meshes, extensive in vitro cultivation is required to obtain a homogenous cell layer covering the mesh. In this context, the objective of this publication is to manufacture meshes made of silk fibres and to enhance the cytoadhesion and cytocompatibility of the biomaterial by surface immobilization of a pro-adhesive wheat germ agglutinin (lectin WGA). We first investigated the affinity between the glycoprotein WGA and cells, in solution and then after covalent immobilization of WGA on silk films. Then, we manufactured meshes made of silk fibres, tailored them with WGA grafting and finally evaluated the cytocompatibility and the inflammatory response of silk and silk–lectin meshes compared to common polypropylene mesh, using fibroblasts and peripheral blood mononuclear cells, respectively. The in vitro experiments revealed that the cytocompatibility of silk can be enhanced by surface immobilization with lectin WGA without exhibiting negative response in terms of pro-inflammatory reaction. Grafting lectin to silk meshes could bring advantages to facilitate cell-coating of meshes prior to implantation, which is an imperative prerequisite for abdominal wall tissue regeneration using cell-based therapy.
Graphical abstract







Similar content being viewed by others
References
Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–70.
Greenwald D, Shumway S, Albear P, Gottlieb L. Mechanical comparison of 10 suture materials before and after in vivo incubation. J Surg Res. 1994;56(4):372–7.
Handley WS. Behandlung der Bauchnarbenbrüche. Langenbecks Arch Klin Chir. 1963;304:296–7.
Fine NA, Lehfeldt M, Gross JE, Downey S, Kind GM, Duda G, et al. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast Reconstr Surg. 2015;135(2):339–51.
Horan RL, Bramono DS, Stanley JR, Simmons Q, Chen J, Boepple HE, et al. Biological and biomechanical assessment of a long-term bioresorbable silk-derived surgical mesh in an abdominal body wall defect model. Hernia. 2009;13(2):189–99.
Gross JE, Horan RL, Gaylord M, Olsen RE, McGill LD, García-López JM, et al. An evaluation of SERI® surgical scaffold, a silk-derived bioresorbable device for soft tissue support and repair in an ovine model of two-stage breast reconstruction. Plast Reconstr Surg Adv Online Artic. 2014;134(5):700e–4e.
Haupt J, Garcia-Lopez JM, Chope K. Use of a novel silk mesh for ventral midline hernioplasty in a mare. BMC Vet Res. 2015;11:58.
Clemens MW, Downey S, Agullo F, Lehfeldt MR, Kind GM, Palladino H, et al. Clinical application of a silk fibroin protein biologic scaffold for abdominal wall fascial reinforcement. Plast Reconstr Surg Glob Open. 2014;2(11):e246.
Petter-Puchner AH, Fortelny RH, Gruber-Blum S, Redl H, Dietz U. The future of stem cell therapy in hernia and abdominal wall repair. Hernia. 2015;19(1):25–31.
Lai JY, Chang PY, Lin JN. Body wall repair using small intestinal submucosa seeded with cells. J Pediatr Surg. 2003;38(12):1752–5.
Conconi MT, De Coppi P, Bellini S, Zara G, Sabatti M, Marzaro M, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26(15):2567–74.
De Coppi P, Bellini S, Conconi MT, Sabatti M, Simonato E, Gamba PG, et al. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12(7):1929–36.
Ayele T, Zuki AB, Noorjahan BM, Noordin MM. Tissue engineering approach to repair abdominal wall defects using cell-seeded bovine tunica vaginalis in a rabbit model. J Mater Sci Mater Med. 2010;21(5):1721–30.
Drewa T, Galazka P, Prokurat A, Wolski Z, Sir J, Wysocka K, et al. Abdominal wall repair using a biodegradable scaffold seeded with cells. J Pediatr Surg. 2005;40(2):317–21.
Dolce CJ, Stefanidis D, Keller JE, Walters KC, Newcomb WL, Heath JJ, et al. Pushing the envelope in biomaterial research: initial results of prosthetic coating with stem cells in a rat model. Surg Endosc. 2010;24(11):2687–93.
Zhao Y, Zhang Z, Wang J, Yin P, Zhou J, Zhen M, et al. Abdominal hernia repair with a decellularized dermal scaffold seeded with autologous bone marrow-derived mesenchymal stem cells. Artif Organs. 2012;36(3):247–55.
Song Z, Peng Z, Liu Z, Yang J, Tang R, Gu Y. Reconstruction of abdominal wall musculofascial defects with small intestinal submucosa scaffolds seeded with tenocytes in rats. Tissue Eng Part A. 2013;19(13–14):1543–53.
Altman AM, Abdul Khalek FJ, Alt EU, Butler CE. Adipose tissue-derived stem cells enhance bioprosthetic mesh repair of ventral hernias. Plast Reconstr Surg. 2010;126(3):845–54.
Iyyanki TS, Dunne LW, Zhang Q, Hubenak J, Turza KC, Butler CE. Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue Eng Part A. 2015;21(3–4):475–85.
Mestak O, Matouskova E, Spurkova Z, Benkova K, Vesely P, Mestak J, et al. Mesenchymal stem cells seeded on cross-linked and noncross-linked acellular porcine dermal scaffolds for long-term full-thickness hernia repair in a small animal model. Artif Organs. 2014;38(7):572–9.
Guillaume O, Teuschl AH, Gruber-Blum S, Fortelny RH, Redl H, Petter-Puchner A. Emerging trends in abdominal wall reinforcement: bringing bio-functionality to meshes. Adv Healthc Mater. 2015;4(12):1763–89.
Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6(10):1612–31.
Cobb WS, Burns JM, Peindl RD, Carbonell AM, Matthews BD, Kercher KW, et al. Textile analysis of heavy weight, mid-weight, and light weight polypropylene mesh in a porcine ventral hernia model. J Surg Res. 2006;136(1):1–7.
Yamada H, Nakao H, Takasu Y, Tsubouchi K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater Sci Eng, C. 2001;14:41–6.
Teuschl AH, Neutsch L, Monforte X, Runzler D, van Griensven M, Gabor F, et al. Enhanced cell adhesion on silk fibroin via lectin surface modification. Acta Biomater. 2014;10(6):2506–17.
Ekwall B, Silano V, Paganuzzi-Stammati A, Zucco F. Toxicity tests with mammalian cell cultures. In: Bourdeau P, Somers E, Richardson GM, Hickman JR, editors. Short-term toxicity tests for non-genotoxic effects. New York: Wiley; 1990. p. 75–98.
Hossain KS, Ochi A, Ooyama E, Magoshi J, Nemoto N. Dynamic light scattering of native silk fibroin solution extracted from different parts of the middle division of the silk gland of the Bombyx mori silkworm. Biomacromolecules. 2003;4(2):350–9.
Pott PP, Schwarz ML, Gundling R, Nowak K, Hohenberger P, Roessner ED. Mechanical properties of mesh materials used for hernia repair and soft tissue augmentation. PLoS ONE. 2012;7(10):e46978.
Kapischke M, Prinz K, Tepel J, Tensfeldt J, Schulz T. Precoating of alloplastic materials with living human fibroblasts–a feasibility study. Surg Endosc. 2005;19(6):791–7.
Sannino A, Conversano F, Esposito A, Maffezzoli A. Polymeric meshes for internal sutures with differentiated adhesion on the two sides. J Mater Sci Mater Med. 2005;16(4):289–96.
Colin Hughes R. Lectins as cell adhesion molecules. Curr Opin Struct Biol. 1992;2:687–92.
KleinJan GH, Buckle T, van Willigen DM, van Oosterom MN, Spa SJ, Kloosterboer HE, et al. Fluorescent lectins for local in vivo visualization of peripheral nerves. Molecules. 2014;19(7):9876–92.
Cheung RC, Wong JH, Pan W, Chan YS, Yin C, Dan X, et al. Marine lectins and their medicinal applications. Appl Microbiol Biotechnol. 2015;99(9):3755–73.
Wittmann V, Pieters RJ. Bridging lectin binding sites by multivalent carbohydrates. Chem Soc Rev. 2013;42(10):4492–503.
Gabor F, Stangl M, Wirth M. Lectin-mediated bioadhesion: binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. J Control Release. 1998;55(2–3):131–42.
Wirth M, Gerhardt K, Wurm C, Gabor F. Lectin-mediated drug delivery: influence of mucin on cytoadhesion of plant lectins in vitro. J Control Release. 2002;79(1–3):183–91.
Klinge U, Klosterhalfen B, Muller M, Anurov M, Ottinger A, Schumpelick V. Influence of polyglactin-coating on functional and morphological parameters of polypropylene-mesh modifications for abdominal wall repair. Biomaterials. 1999;20(7):613–23.
Orenstein SB, Qiao Y, Kaur M, Klueh U, Kreutzer DL, Novitsky YW. Human monocyte activation by biologic and biodegradable meshes in vitro. Surg Endosc. 2010;24(4):805–11.
Orenstein SB, Qiao Y, Klueh U, Kreutzer DL, Novitsky YW. Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia. 2010;14(4):401–7.
Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng. 2009;107(5):556–61.
Lis H, Sharon N. Biological properties of lectins. In: Irvin Liener E, Sharon N, Goldstein IJ, editors. The lectins, properties, functions, and applications in biology and medicine. Orlando: Academic Press; 1986. p. 265–91.
Acknowledgments
This work was supported with resources/facilities of the Ludwig Boltzmann Institute of Experimental and Clinical Traumatology in Vienna and the FemTech grant programme. The financial support by the City of Vienna (MA 27, Project 12-06 and MA 23, Project 14-06) is gratefully acknowledged. Furthermore, we would like to thank M. Jafarmadar, A. Klotz and A. Khadem for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10856_2015_5648_MOESM1_ESM.tif
Illustration of the silk yarn and of the macroporous silk mesh obtained after knitting. Macroscopic observation of the silk yarn (A) and of the silk mesh obtained after knitting using a Silver Reed® machine (B). Inlet displays a high magnification of the mesh obtained by SEM (Magnification 20x, scale bar represents 1 mm) (C). Supplementary material 1 (TIFF 1134 kb)
10856_2015_5648_MOESM2_ESM.tif
Illustrations of the silk fibres before and after treatment. Macroscopic (A) and microscopic pictures of silk fibres before treatment (B), after degumming in boiling Na2CO3 (C) and after degumming followed by autoclave sterilization (D). Supplementary material 2 (TIFF 4770 kb)
Rights and permissions
About this article
Cite this article
Guillaume, O., Park, J., Monforte, X. et al. Fabrication of silk mesh with enhanced cytocompatibility: preliminary in vitro investigation toward cell-based therapy for hernia repair. J Mater Sci: Mater Med 27, 37 (2016). https://doi.org/10.1007/s10856-015-5648-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5648-3