Abstract
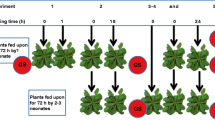
Glucosinolates are a diverse group of defensive secondary metabolites that is characteristic of the Brassicales. Arabidopsis thaliana (L.) Heynh. (Brassicaceae) lines with mutations that greatly reduce abundance of indole glucosinolates (cyp79B2 cyp79B3), aliphatic glucosinolates (myb28 myb29), or both (cyp79B2 cyp79B3 myb28 myb29) make it possible to test the in vivo defensive function of these two major glucosinolate classes. In experiments with Lepidoptera that are not crucifer-feeding specialists, aliphatic and indole glucosinolates had an additive effect on Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) larval growth, whereas Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) and Manduca sexta (L.) (Lepidoptera: Sphingidae) were affected only by the absence of aliphatic glucosinolates. In the case of two crucifer-feeding specialists, Pieris rapae (L.) (Lepidoptera: Pieridae) and Plutella xylostella (L.) (Lepidoptera: Plutellidae), there were no major changes in larval performance due to decreased aliphatic and/or indole glucosinolate content. Nevertheless, choice tests show that aliphatic and indole glucosinolates act in an additive manner to promote larval feeding of both species and P. rapae oviposition. Together, these results support the hypothesis that a diversity of glucosinolates is required to limit the growth of multiple insect herbivores.





Similar content being viewed by others
References
Barth, C., and Jander, G. 2006. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46:549–562.
Beekwilder, J., Van Leeuwen, W., Van Dam, N. M., Bertossi, M., Grandi, V., Mizzi, L., Soloviev, M., Szabados, L., Molthoff, J. W., Schipper, B., Verbocht, H., De Vos, R. C., Morandini, P., Aarts, M. G., and Bovy, A. 2008. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 3:e2068.
Bidart-Bouzat, M. G., and Kliebenstein, D. J. 2008. Differential levels of insect herbivory in the field associated with genotypic variation in glucosinolates in Arabidopsis thaliana. J. Chem. Ecol. 34:1026–1037.
Bidart-Bouzat, M. G., Mithen, R., and Berenbaum, M. R. 2005. Elevated CO(2) influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 145:415–424.
Brader, G., Tas, E., and Palva, E. T. 2001. Jasmonate-dependent induction of indole glucosinolates in Arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol. 126:849–60.
Burow, M., Müller, R., Gershenzon, J., and Wittstock, U. 2006. Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J. Chem. Ecol. 32:2333–49.
De Vos, M., and Jander, G. 2009. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Env. 32:1548–1560.
De Vos, M., Kriksunov, K., and Jander, G. 2008. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae (white cabbage butterfly). Plant Physiol. 146:916–926.
Del Campo, M. L., Smedley, S. R., and Eisner, T. 2005. Reproductive benefits derived from defensive plant alkaloid possession in an arctiid moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. U S A 102:13508–12.
Fahey, J. W., Zalcmann, A. T., and Talalay, P. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51.
Gigolashvili, T., Berger, B., Mock, H. P., Muller, C., Weisshaar, B., and Flugge, U. I. 2007a. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 56:886–890.
Gigolashvili, T., Yatusevich, R., Berger, B., Muller, C., and Flugge, U. I. 2007b. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 51:247–261.
Glawischnig, E., Hansen, B. G., Olsen, C. E., and Halkier, B. A. 2004. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. U S A 101:8245–50.
Gupta, P. D., and Thorsteinson, A. J. 1960. Food plant relationship of the diamond-back moth (Plutella maculipennis (Curt.)) I. Gustation and olfaction in relation to botanical specificity of the larva. Ent. Exp. Appl. 3:241–250.
Halkier, B. A., and Gershenzon, J. 2006. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57:303–333.
Hansen, B. G., Kliebenstein, D. J., and Halkier, B. A. 2007. Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 50:902–10.
Jander, G., Cui, J., Nhan, B., Pierce, N. E., and Ausubel, F. M. 2001. The TASTY locus on chromosome 1 of Arabidopsis affects feeding of the insect herbivore Trichoplusia ni. Plant Physiol. 126:890–8.
Kim, J. H., and Jander, G. 2007. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 49:1008–1019.
Kim, J. H., Lee, B. W., Schroeder, F. C., and Jander, G. 2008. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 54:1015–1026.
Kliebenstein, D. J., Kroymann, J., Brown, P., Figuth, A., Pedersen, D., Gershenzon, J., and Mitchell-olds, T. 2001. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126:811–825.
Lambrix, V., Reichelt, M., Mitchell-olds, T., Kliebenstein, D. J., and Gershenzon, J. 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13:2793–807.
Mauricio, R. 1998. Costs of resistance to natural enemies in field popualtions of the annual plant Arabidopsis thaliana. Am. Nat. 151:20–28.
Mauricio, R., and Rausher, M. D. 1997. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51:1435–1444.
Mewis, I., Appel, H. M., Hom, A., Raina, R., and Schultz, J. C. 2005. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138:1149–62.
Miles, C. I., Del Campo, M. L., and Renwick, J. A. 2005. Behavioral and chemosensory responses to a host recognition cue by larvae of Pieris rapae. J. Comp. Phys. A 191:147-155.
Pegadaraju, V., Knepper, C., Reese, J., and Shah, J. 2005. Premature leaf senescence modulated by the Arabidopsis PHYTOALEXIN DEFICIENT4 gene is associated with defense against the phloem-feeding green peach aphid. Plant Physiol. 139:1927–34.
Pfalz, M., Vogel, H., and Kroymann, J. 2009. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 21:985–99.
Rasband, W. S. 1997–2007. ImageJ. National Institutes of Health, Bethesda, Maryland, USA http://rsb.info.nih.gov/ij/.
Ratzka, A., Vogel, H., Kliebenstein, D. J., Mitchell-olds, T., and Kroymann, J. 2002. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. U S A 99:11223–11228.
Reichelt, M., Brown, P. D., Schneider, B., Oldham, N. J., Stauber, E., Tokuhisa, J., Kliebenstein, D. J., Mitchell-olds, T., and Gershenzon, J. 2002. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 59:663–671.
Renwick, J. A. A., Radke, C. D., Sachdev-gupta, K., and Städler, E. 1992. Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 3:33–38.
Reymond, P., Bodenhausen, N., Van Poecke, R. M., Krishnamurthy, V., Dicke, M., and Farmer, E. E. 2004. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16:3132–3147.
Rohr, F., Ulrichs, C., Mucha-pelzer, T., and Mewis, I. 2006. Variability of aliphatic glucosinolates in Arabidopsis and their influence on insect resistance. Commun. Agric. Appl. Biol. Sci. 71:507–15.
Sarfraz, M., Dosdall, L. M., and Keddie, B. A. 2006. Diamondback moth-host plant interactions: Implications for pest management. Crop Protection 25:625–639.
Sarosh, B. R., Wittstock, U., Halkier, B. A., and Ekbom, B. 2010. The influence of metabolically engineered glucosinolates profiles in Arabidopsis thaliana on Plutella xylostella preference and performance. Chemoecology 20:1–9.
Schlaeppi, K., Bodenhausen, N., Buchala, A., Mauch, F., and Reymond, P. 2008. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates ad is more susceptible to the insect herbivore Spodoptera litoralis. Plant J. 55:774–786.
Sønderby, I. E., Hansen, B. G., Bjarnholt, N., Ticconi, C., Halkier, B. A., and Kliebenstein, D. J. 2007. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2:e1322.
Städler, E., Renwick, J. A. A., Radke, C. D., and Sachdev-gupta, K. 1995. Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris rapae. Physiol. Entomol. 20:175.
Sun, J. Y., Sønderby, I. E., Halkier, B. A., Jander, G., and De Vos, M. 2009. Non-volatile intact indole glucosinolates are reliable host recognition cues for Plutella xylostella oviposition. J. Chem. Ecol. 35:1427–1436.
Thorsteinson, A. J. 1953. The chemotactic responses that determine host specificity in an oligophagous insect (Plutella maculipennis (Curt.) Lepidoptera). Can. J. Zool. 31:52–72.
Van Loon, J. J. A., Wang, C. Z., Nielsen, J. K., Gols, R., and Qiu, Y. T. 2002. Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: Chemoreception and behaviour. Ent. Exp. Appl. 104:27–43.
Verschaffelt, E. 1910. The cause determining the selection of food in some herbivorous insects. Proc. R. Acad. Amst. 13:536–542.
Wittstock, U., and Halkier, B. A. 2002. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 7:263–270.
Wittstock, U., and Burow, M. 2010. Glucosinolate breakdown in Arabidopsis—mechanism, regulation and biological significance. The Arabidopsis Book. Rockville: American Society of Plant Biologists. doi:10.1199/tab.0134.
Wittstock, U., Agerbirk, N., Stauber, E. J., Olsen, C. E., Hippler, M., Mitchell-olds, T., Gershenzon, J., and Vogel, H. 2004. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. U S A 101:4859–64.
Zhao, Y., Hull, A. K., Gupta, N. R., Goss, K. A., Alonso, J., Ecker, J. R., Normanly, J., Chory, J., and Celenza, J. L. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 16:3100–12.
Acknowledgements
This research was funded by the VKR-Research Centre for Pro-Active Plants (Villum Kahn Rasmussen’s foundation) to B.A.H., and National Science Foundation award #IOS-0718733 to G.J. We thank M. del Campo and J. Beal for providing M. sexta eggs, L. Meihls for assisting with P. xylostella experiments, and L. Heise for helping to maintain the P. rapae culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, R., de Vos, M., Sun, J.Y. et al. Differential Effects of Indole and Aliphatic Glucosinolates on Lepidopteran Herbivores. J Chem Ecol 36, 905–913 (2010). https://doi.org/10.1007/s10886-010-9825-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9825-z