Abstract
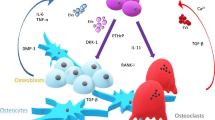
The most common skeletal complication of breast cancer is osteolytic bone metastasis. Bone metastases are present in 80% of patients with advanced disease and cause significant morbidity. They are most often osteolytic, but can be osteoblastic or mixed. Tumor cells, osteoblasts, osteoclasts and bone matrix are the four components of a vicious cycle necessary for the initiation and development of bone metastases. Tumor cell gene expression is modified by interaction with bone-derived factors. For example, parathyroid hormone related protein (PTHrP), a tumor cell factor, is upregulated by bone-derived transforming growth factor β (TGFβ). Tumor cell factors, in turn, act upon bone cells to cause dysregulated bone destruction and formation. PTHrP increases osteoblast expression of RANK (receptor activator of NFκB) ligand which, in turn, activates osteoclasts. PTHrP-independent osteolytic factors, such as interleukin [IL]-11 and IL-8, also contribute to the vicious cycle. Other tumor-bone interactions, such as stimulation of tumor-homing through the CXCR4 chemokine receptor by its bone-derived ligand stromal-derived factor-1 (SDF-1), may be responsible for the site-specific predilection of breast cancer for bone. These factors and their roles in fueling the vicious cycle may identify novel targets for therapies to prevent metastasis.
Similar content being viewed by others
Abbreviations
- AI:
-
aromatase inhibitor
- BMP:
-
bone morphogenetic protein
- CT:
-
computed tomography
- CTGF:
-
connective tissue growth factor
- EGF:
-
ET-epidermal growth factor
- 1:
-
endothelin-1
- FGF:
-
fibroblast growth factor
- HHM:
-
hypercalcemia of malignancy
- IGF:
-
insulin-like growth factor
- IL:
-
interleukin
- LPA:
-
lysophosphatidic acid
- MAP:
-
M-mitogen-activated protein
- CSF:
-
macrophage colony-stimulating factor
- MMP:
-
matrix metalloproteinase
- MRI:
-
magnetic resonance imaging
- NFκB:
-
nuclear factor kappa B
- OPG:
-
osteoprotegerin
- PCR:
-
polymerase chain reaction
- PDGF:
-
platelet-derived growth factor
- PET:
-
positron emission tomography
- PTH:
-
parathyroid hormone
- PTHrP:
-
parathyroid hormone-related protein
- RANK:
-
receptor activator of NFκB
- SDF:
-
stromal-derived factor
- SERM:
-
selective estrogen-receptor modulator
- SPECT:
-
single photon emission computed tomography
- SRE:
-
skeletal-related event
- TGF:
-
transforming growth factor
- TNF:
-
tumor necrosis factor
- VEGF:
-
vascular endothelial growth factor
References
Mundy GR, Bertolini DR. Bone destruction and hypercalcemia in plasma cell myeloma. Semin Oncol 1986;13:291–9.
Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer 2000;88(12 Suppl):2892–8.
Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 1989;8:98–101.
Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003;3:453–8.
Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584–93.
Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol 2003;34:646–53.
Guise TA, Mundy GR. Cancer and bone. Endocr Rev 1998;19:18–54.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61–6.
Boxer DI, Todd CE, Coleman R, Fogelman I. Bone secondaries in breast cancer: The solitary metastasis. J Nucl Med 1989;30:1318–20.
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol 2004;22:2942–53.
Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Metastatic breast cancer confined to the skeletal system. An indolent disease. Am J Med 1986;81:381–6.
Singletary SE, Walsh G, Vauthey JN, Curley S, Sawaya R, Weber KL, et al. A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 2003;8:241–51.
Fontana A, Delmas PD. Markers of bone turnover in bone metastases. Cancer 2000;88(12 Suppl):2952–60.
Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer 2000;82:858–64.
Koizumi M, Ogata E. Bone metabolic markers as gauges of metastasis to bone: A review. Ann Nucl Med 2002;16:161–8.
Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002;99:397–406.
Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer 2002;2:201–9.
Arguello F, Baggs RB, Frantz CN. A murine model of experimental metastasis to bone and bone marrow. Cancer Res 1998;48:6876–81.
Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000;6:521–8.
Clohisy DR, Mantyh PW. Bone cancer pain and the role of RANKL/OPG. J Musculoskelet Neuronal Interact 2004;4:293–300.
Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA 2003;100:10954–9.
Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, et al. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 2004;126:1043–52.
Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–71.
Yoneda T. Cellular and molecular basis of preferential metastasis of breast cancer to bone. J Orthop Sci 2000;5:75–81.
Lipton A. Bone metastases in breast cancer. Curr Treat Options Oncol 2003;4:151–8.
Orr W, Varani J, Gondex MK, Ward PA, Mundy GR. Chemotactic responses of tumor cells to products of resorbing bone. Science 1979;203:176–9.
Ozbas S, Dafydd H, Purushotham AD. Bone marrow micrometastasis in breast cancer. Br J Surg 2003;90:290–301.
Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev 2003;83:337–76.
Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer 1997;80:S37.
Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation 2002;70:561–73.
Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 1999;103:517–24.
Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002;295:2387–92.
Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat Rev Cancer 2002;2:657–72.
Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375–9.
Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metastasis 2003;20:275–84.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6.
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537–49.
Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res 2002;62:7203–6.
Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res 2005;20:318–29.
Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 2003;97(3 Suppl):739–47.
Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 2001;98:1853–8.
Teti A, Migliaccio S, Baron R. The role of the alphaVbeta3 integrin in the development of osteolytic bone metastases: A pharmacological target for alternative therapy? Calcif Tissue Int 2002;71:293–9.
Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005;115:44–55.
Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR. Scanning electron microscopy in bone pathology: Review of methods, potential and applications. Scanning Electron Microsc 1986;4:4–54.
Taube T, Elomaa I, Blomqvist C, Beneton MN, Kanis JA. Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone 1994;15:161–6.
Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ, et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA 1987;84:5048–52.
Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, et al. Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem 1987;262:7151–6.
Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC, et al. Parathyroid hormone-like protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest 1987;80:1803–7.
Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, Diefenbach-Jagger H, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: Cloning and expression. Science 1987;237:893–6.
Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: A single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 1992;89:2732–6.
Horiuchi N, Caulfield MP, Fisher JE, Goldman ME, McKee RL, Reagan JE, et al. Similarity of synthetic peptide from human tumor to parathyroid hormone in vivo and in vitro. Science 1987;238:1566–8.
Burtis WJ, Brady TG, Orloff JJ, Ersbak JB, Warrell RP, Jr, Olson BR, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med 1990;322:1106–12.
Bundred NJ, Ratcliffe WA, Walker RA, Coley S, Morrison JM, Ratcliffe JG. Parathyroid hormone related protein and hypercalcaemia in breast cancer. BMJ 1991;303:1506–9.
Bundred NJ, Walker RA, Ratcliffe WA, Warwick J, Morrison JM, Ratcliffe JG. Parathyroid hormone related protein and skeletal morbidity in breast cancer. Eur J Cancer 1992;28:690–2.
Southby J, Kissin MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res 1990;50:7710–6.
Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 1999;140:4451–8.
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997;390:175–9.
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999;20:345–57.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–76.
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998;139:1329–37.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998;95:3597–602.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997;89:309–19.
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544–9.
Henderson MA, Danks JA, Moseley JM, Slavin JL, Harris TL, McKinlay MR, et al. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J Natl Cancer Inst 2001;93:234–7.
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Res 1991;51:3059–61.
Vargas SJ, Gillespie MT, Powell GJ, Southby J, Danks JA, Moseley JM, et al. Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J Bone Miner Res 1992;7:971–9.
Iguchi H, Tanaka S, Ozawa Y, Kashiwakuma T, Kimura T, Hiraga T, et al. An experimental model of bone metastasis by human lung cancer cells: The role of parathyroid hormone-related protein in bone metastasis. Cancer Res 1996;56:4040–3.
Buchs N, Manen D, Bonjour JP, Rizzoli R. Calcium stimulates parathyroid hormone-related protein production in Leydig tumor cells through a putative cation-sensing mechanism. Eur J Endocrinol 2000;142:500–5.
Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Brown EM. Extracellular calcium-sensing receptor (CaR) expression and its potential role in parathyroid hormone-related peptide (PTHrP) secretion in the H-500 rat Leydig cell model of humoral hypercalcemia of malignancy. Biochem Biophys Res Commun 2000;269:427–32.
Yamaguchi T, Chattopadhyay N, Brown EM. G protein-coupled extracellular Ca2+ (Ca2+o)-sensing receptor (CaR): Roles in cell signaling and control of diverse cellular functions. Adv Pharmacol 2000;47:209–53.
Nemeth EF. Pharmacological regulation of parathyroid hormone secretion. Curr Pharm Des 2002;8:2077–87.
van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Lowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: Elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res 2001;16:1077–91.
Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res 2002;62:5571–9.
Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone: It is not all about PTHrP. Clin Orthop 2003;415:S39–45.
Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 2004;114:1714–25.
Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem 1986;261:12665–74.
Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999;103:197–206.
Wakefield LM, Roberts AB. TGF-beta signaling: Positive and negative effects on tumorigenesis. Curr Opin Genet Dev 2002;12:22–9.
Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, et al. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 2002;277:24571–8.
Coleman RE. Bisphosphonates for the prevention of bone metastases. Semin Oncol 2002;29(6 Suppl 21):43–9.
Coleman RE. Bisphosphonates: Clinical experience. Oncologist 2004;9:14–27.
Coleman RE. The role of bisphosphonates in breast cancer. Breast 2004;13:S19–28.
Lipton A. Toward new horizons: the future of bisphosphonate therapy. Oncologist 2004;9:38–47.
Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 2000;15:2211–21.
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459–68.
Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res 1997;57:3890–4.
van der Pluijm G, Vloedgraven H, van Beek E, Wee-Pals L, Lowik C, Papapoulos S. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest 1996;98:698–705.
van der Pluijm G, Vloedgraven HJ, Ivanov B, Robey FA, Grzesik WJ, Robey PG, et al. Bone sialoprotein peptides are potent inhibitors of breast cancer cell adhesion to bone. Cancer Res 1996;56:1948–55.
Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002;62:6538–44.
Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res 2004;10:4559–67.
Diel IJ, Solomayer EF, Bastert G. Treatment of metastatic bone disease in breast cancer: Bisphosphonates. Clin Breast Cancer 2000;1:43–51.
Diel IJ, Solomayer EF, Bastert G. Bisphosphonates and the prevention of metastasis: First evidences from preclinical and clinical studies. Cancer 2000;88(12 Suppl):3080–8.
Shakespeare W, Yang M, Bohacek R, Cerasoli F, Stebbins K, Sundaramoorthi R, et al. Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc Natl Acad Sci USA 2000;97:9373–8.
Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 2001;61:4432–6.
Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 2003;97(3 Suppl):887–92.
Gallwitz WE, Guise TA, Mundy GR. Guanosine nucleotides inhibit different syndromes of PTHrP excess caused by human cancers in vivo. J Clin Invest 2002;110:1559–72.
Funk JL, Wei H. Regulation of parathyroid hormone-related protein expression in MCF-7 breast carcinoma cells by estrogen and antiestrogens. Biochem Biophys Res Commun 1998;251:849–54.
Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: A double-blind, placebo-controlled study. J Clin Oncol 1997;15:955–62.
Coleman RE. Hormone- and chemotherapy-induced bone loss in breast cancer. Oncology 2004;18:16–20.
Harvey HA. Optimizing bisphosphonate therapy in patients with breast cancer on endocrine therapy. Semin Oncol 2004;31:23–30.
Coleman RE. Current and future status of adjuvant therapy for breast cancer. Cancer 2003;97(3 Suppl):880–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kozlow, W., Guise, T.A. Breast Cancer Metastasis to Bone: Mechanisms of Osteolysis and Implications for Therapy. J Mammary Gland Biol Neoplasia 10, 169–180 (2005). https://doi.org/10.1007/s10911-005-5399-8
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-5399-8