Abstract
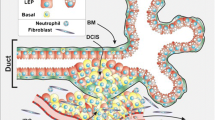
Host cellular paracrine regulation of tumor progression is an important determinant of tumor biology but one cell that has been ignored in this regulation is the myoepithelial cell. Myoepithelial cells surround normal ducts and precancerous lesions, especially of the breast and form a natural border separating proliferating epithelial cells from proliferating endothelial cells (angiogenesis). Myoepithelial cells may thus negatively regulate tumor invasion and metastasis. Whereas epithelial cells are susceptible targets for transforming events, myoepithelial cells are resistant. Therefore, it can be said that myoepithelial cells function as both autocrine as well as paracrine tumor suppressors. Our laboratory has found that myoepithelial cells secrete a number of suppressor molecules including high amounts of diverse proteinase inhibitors and angiogenic inhibitors but low amounts of proteinases and angiogenic factors compared to common malignant cell lines. This observation has been made in vitro, in mice, and in humans and suggests that myoepithelial cells exert pleiotropic suppressive effects on tumor progression. The gene expression profile of myoepithelial cells may explain the pronounced anti-invasive and anti-angiogenic effects of myoepithelial cells on carcinoma cells and may also account for the reduced malignancy of myoepithelial tumors, which are devoid of appreciable angiogenesis and invasive behavior.
Similar content being viewed by others
Abbreviations
- aFGF:
-
acidic fibroblast growth factor
- α1-AT:
-
alpha 1-antitrypsin
- 5-azaC:
-
5-azacytidine
- bFGF:
-
basic fibroblast growth factor
- CALLA:
-
common acute lymphocytic leukemia antigen
- CAT:
-
chloramphenicol acetyl transferase
- CGH:
-
comparative genomic hybridization
- COL1A1:
-
type I collagen α1 chain
- COL4A1:
-
type IV collagen α1 chain
- COL4A2:
-
type IV collagen α2 chain
- CM:
-
conditioned medium
- dB-cAMP:
-
N6,2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate
- DCIS:
-
ductal carcinoma in situ
- DF:
-
ductal lavage fluid
- DMSO:
-
dimethylsulfoxide
- EHS:
-
Engelbreth-Holm-Swarm
- ER-α:
-
estrogen receptor-α
- ER-β:
-
estrogen receptor-β
- FCS:
-
fetal calf serum
- FN1:
-
fibronectin
- HB-ECGF:
-
heparin-binding endothelial cell growth factor
- HGF:
-
hepatocyte growth factor
- HIF-1α:
-
hypoxia-inducible factor-alpha
- HMEC:
-
human mammary epithelial cells
- HRE:
-
hypoxia response element
- HRT:
-
hormone replacement therapy
- HSPG2:
-
perlecan
- iNOS:
-
inducible nitric oxide synthase
- K-SFM:
-
keratinocyte serum-free medium
- LOH:
-
loss of heterozygosity
- MMP-9:
-
matrix metalloproteinase-9
- MUC-1:
-
mucin-1
- Na-But:
-
sodium butyrate
- PAI-1:
-
plasminogen activator inhibitor-1
- PD-ECGF:
-
platelet-derived endothelial cell growth factor
- PlGF:
-
placental growth factor
- PMA:
-
phorbol 12-myristate 13-acetate: PN-II, protease nexin-II
- RA:
-
all trans retinoic acid
- TIMP-1:
-
tissue inhibitor of metalloproteinase-1
- TFGα:
-
transforming growth factor α
- TGFβ:
-
transforming growth factor β
- TNFα:
-
tumor necrosis factor α
- UVE:
-
human umbilical vein endothelial cells
- uPA:
-
urokinase-type plasminogen activator
- VEGF:
-
vascular endothelial growth factor
- vWf:
-
von Willebrand factor
References
Cavenee WK. A siren song from tumor cells. J Clin Invest 1993;91:3.
Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991;64:327–36.
Safarians S, Sternlicht MD, Freiman CJ, Huaman JA, Barsky SH. The primary tumor is the primary source of metastasis in a human melanoma/SCID model: implications for the direct autocrine and paracrine epigenetic regulation of the metastatic process. Int J Cancer 1996;66:151–8.
Folkman J, Klagsbrun M. Angiogenic factors. Science 1987;235:442–7.
Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel RS. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci USA 1991;88:6028–32.
Sgroi DC, Teng S, Robinson G, Le Vangie R, Hudson JR, Elkahloun AG. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res 1999;59:5656–61.
Guelstein VI, Tchypsheva TA, Ermilova VD, Ljubimov AV. Myoepithelial and basement membrane antigens in benign and malignant human breast tumors. Int J Cancer 1993;53:269–77.
Cutler LS. The role of extracellular matrix in the morphogenesis and differentiation of salivary glands. Adv Dent Res 1990;4:27–33.
Sternlicht MD, Safarians S, Calcaterra TC, Barsky SH. Establishment and characterization of a novel human myoepithelial cell line and matrix-producing xenograft from a parotid basal cell adenocarcinoma. In Vitro Cell Dev Biol 1996;32:550–63.
Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997;3:1949–58.
Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Barsky SH. The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene 2000;19:3449–59.
Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med 2000;6:196–9.
Shao ZM, Radziszewski WJ, Barsky SH. Tamoxifen enhances myoepithelial cell suppression of human breast carcinoma progression by two different effector mechanisms. Cancer Lett 2000;157:133–44.
Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN. Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol 1990;68:1–19.
Albini A, Iwamoto Y, Kleinman HK, Martin GS, Aaronson SA, Kozlowski JM, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987;47:3239–45.
Kataoka H, Seguchi K, Iwamura T, Moriyama T, Nabeshima K, Koono M. Reverse-zymographic analysis of protease nexin-II/amyloid b protein precursor of human carcinoma cell lines, with special reference to the grade of differentiation and metastatic phenotype. Int J Cancer 1995;60:123–8.
Narindrasorasak S, Lowery DE, Altman RA, Gonzalez-DeWhitt PA, Greenberg BD, Kisilevsky R. Characterization of high affinity binding between laminin and Alzheimer’s disease amyloid precursor proteins. Lab Invest 1992;67:643–52.
Rao CN, Liu YY, Peavey CL, Woodley DT. Novel extracellular matrix-associated serine proteinase inhibitors from human skin fibroblasts. Arch Biochem Biophys 1995;317:311–4.
Zou Z, Anisowicz A, Hendrix MJC, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994;263:526–9.
Hopkins PCR, Whisstock J. Function of maspin. Science 1994;265:1893–4.
Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, et al. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases: evidence that maspin is not a protease inhibitory serpin. J Biol Chem 1995;270:15832–7.
Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem 1994;269:30988–93.
Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757–63.
Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J 1996;10:1183–91.
Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 1994;54:6504–11.
Dairkee SH, Puett L, Hackett AJ. Expression of basal and luminal epithelial-specific keratins on normal, benign and malignant breast tissue. J Natl Cancer Inst 1988;80:691–5.
Emerman JT, Wilkinson DA. Routine culturing of normal, dysplastic and malignant human mammary epithelial cells from small tissue samples. In Vitro Cell Dev Biol 1990;26:1186–94.
Emerman JT, Eaves CJ. Lack of effect of hematopoietic growth factors on human breast epithelial cell growth in serum-free primary culture. Bone Marrow Transplant 1994;13:285–91.
Rudland PS. Histochemical organization and cellular composition of ductal buds in developing human breast: evidence of cytochemical intermediate between epithelial and myoepithelial cells. J Histochem Cytochem 1991;39:1471–84.
O’Hare MJ, Ormerod MG, Monaghan P, Lane EB, Gusterson, BA. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation 1991;46:209–21.
Kedeshian P, Sternlicht MD, Nguyen M, Shao ZM, Barsky SH. Humatrix, a novel myoepithelial matrical gel with unique biochemical and biological properties. Cancer Lett 1998;123:215–26.
Barsky SH. Myoepithelial mRNA expression profiling reveals a common tumor-suppressor phenotype. Exp Mol Pathol 2003;74:113–22.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumors. Nature 2000;406:747–52.
Sorlie T, Perou CM, Tibshirini R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barsky, S.H., Karlin, N.J. Myoepithelial Cells: Autocrine and Paracrine Suppressors of Breast Cancer Progression. J Mammary Gland Biol Neoplasia 10, 249–260 (2005). https://doi.org/10.1007/s10911-005-9585-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-9585-5