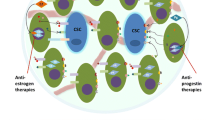
Abstract
From a developmental point of view, tumors can be seen as aberrant versions of their tissue of origin. For example, tumors often partially retain differentiation markers of their tissue of origin and there is evidence that they contain cancer stem cells (CSCs) that drive tumorigenesis. In this review, we summarise current evidence that breast CSCs may partly explain endocrine resistance in breast cancer. In normal breast, the stem cells are known to possess a basal phenotype and to be mainly ERα−. If the hierarchy in breast cancer reflects this, the breast CSC may be endocrine resistant because it expresses very little ERα and can only respond to treatment by virtue of paracrine influences of neighboring, differentiated ERα+ tumor cells. Normal breast epithelial stem cells are highly dependent on the EGFR and other growth factor receptors and it may be that the observed increased growth factor receptor expression in endocrine-resistant breast cancers reflects an increased proportion of CSCs selected by endocrine therapies. There is evidence from a number of studies that breast CSCs are ERα− and EGFR+/HER2+, which would support this view. CSCs also express mesenchymal genes which are suppressed by ERα expression, further indicating the mutual exclusion between ERα+ cells and the CSCs. As we learn more about CSCs, differentiation and the expression and functional activity of the ERα in these cells in diverse breast tumor sub-types, it is hoped that our understanding will lead to new modalities to overcome the problem of endocrine resistance in the clinic.

Similar content being viewed by others
Abbreviations
- CSC:
-
Cancer stem-like cells
- ER:
-
Estrogen receptorα
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial mesenchymal transition
- LN:
-
Lymph node
- ALDH1:
-
Aldehyde dehydrogenase 1
- HDAC:
-
Histone deacetylase
- DNMT:
-
DNA methyl transferase
References
Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature 2006;439(7072):84–8.
Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006;439(7079):993–7.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7.
Kelly PN, et al. Tumor growth need not be driven by rare cancer stem cells. Science 2007;317(5836):337.
Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature 2008;456(7222):593–8.
Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8.
Singh SK, et al. Identification of human brain tumour initiating cells. Nature 2004;432(7015):396–401.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–8.
Collins AT, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51.
O’Brien CA, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445(7123):106–10.
Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445(7123):111–5.
Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–11.
Storms RW, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA. 1999;96(16):9118–23.
Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67.
Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25.
Horwitz KB, et al. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci USA. 2008;105(15):5774–9.
Howell A, Wardley AM. Overview of the impact of conventional systemic therapies on breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S9–16.
Mallepell S, et al. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103(7):2196–201.
Brisken C, et al. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210(1):96–106.
Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127(2):304–15.
Brisken C, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95(9):5076–81.
Keeling JW, et al. Oestrogen receptor alpha in female fetal, infant, and child mammary tissue. J Pathol. 2000;191(4):449–51.
Korach KS, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–86, discussion 186–8.
Russo J, et al. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53(3):217–27.
Clarke RB, et al. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–91.
Asselin-Labat ML, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98(14):1011–4.
Sleeman KE, et al. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176(1):19–26.
Raouf A, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3(1):109–18.
Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73.
Sorlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–23.
Dowsett M, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006;17(5):818–26.
Howell A, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365(9453):60–2.
Gelber RD, et al. Adjuvant chemotherapy plus tamoxifen compared with tamoxifen alone for postmenopausal breast cancer: meta-analysis of quality-adjusted survival. Lancet 1996;347(9008):1066–71.
Giltnane JM, et al. Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J Clin Oncol. 2007;25(21):3007–14.
Knowlden JM, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 2003;144(3):1032–44.
Massarweh S, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66(16):8266–73.
Pancholi S, et al. ERBB2 influences the subcellular localization of the estrogen receptor in tamoxifen-resistant MCF-7 cells leading to the activation of AKT and p90RSK. Endocr Relat Cancer. 2008 Dec;15(4):985–1002.
Sarwar N, et al. Phosphorylation of ERalpha at serine 118 in primary breast cancer and in tamoxifen-resistant tumours is indicative of a complex role for ERalpha phosphorylation in breast cancer progression. Endocr Relat Cancer. 2006;13(3):851–61.
Hiscox S, et al. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97(3):263–74.
Campbell RA, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276(13):9817–24.
Hebbard L, et al. CD44 expression and regulation during mammary gland development and function. J Cell Sci. 2000;113(Pt 14):2619–30.
Farnie G, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99(8):616–27.
Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67.
Korkaya H, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008;27(47):6120–30.
Magnifico A, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are Trastuzumab sensitive. Clin Cancer Res. 2009 (in press).
Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9.
Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007;131(6):1109–23.
Dontu G, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–R615.
Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66(3):1517–25.
Rizzo P, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68(13):5226–35.
Osipo C, et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene 2008;27(37):5019–32.
Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–85.
Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133(4):704–15.
Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118(2):290–301.
Hiscox S, et al. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin Exp Metastasis. 2007;24(3):157–67.
Hiscox S, et al. Dual targeting of Src and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res Treat. 2008 May 21. doi:10.1007/s10549-008-0058-6.
Zhou Y, et al. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59.
Borley AC, et al. Anti-oestrogens but not oestrogen deprivation promote cellular invasion in intercellular adhesion-deficient breast cancer cells. Breast Cancer Res. 2008;10(6):R103.
Hiscox S, Jiang WG. Regulation of endothelial CD44 expression and endothelium-tumour cell interactions by hepatocyte growth factor/scatter factor. Biochem Biophys Res Commun. 1997;233(1):1–5.
Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21(12):2907–18.
Ye Y, et al. ERalpha suppresses slug expression directly by transcriptional repression. Biochem J. 2008;416(2):179–87.
Perou CM, et al. Molecular portraits of human breast tumours. Nature 2000;406(6797):747–52.
Sims AH, et al. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4(9):516–25.
Bloushtain-Qimron N, et al. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci USA. 2008;105(37):14076–81.
Lower EE, et al. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90(1):65–70.
Fehm T, et al. ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 2008;10(5):R76.
Lapidus RG, et al. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res. 1996;2(5):805–10.
Ottaviano YL, et al. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54(10):2552–5.
Badia E, et al. Tamoxifen resistance and epigenetic modifications in breast cancer cell lines. Curr Med Chem. 2007;14(28):3035–45.
Croker AK, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2008 Aug 4 (in press).
Sheridan C, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59.
Ouhtit A, et al. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171(6):2033–9.
Hiscox S, et al. Chronic exposure to fulvestrant promotes overexpression of the c-Met receptor in breast cancer cells: implications for tumour–stroma interactions. Endocr Relat Cancer. 2006;13(4):1085–99.
Hiscox S, et al. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (‘Iressa’, ZD1839). Clin Exp Metastasis. 2004;21(3):201–12.
Mine S, et al. Hepatocyte growth factor enhances adhesion of breast cancer cells to endothelial cells in vitro through up-regulation of CD44. Exp Cell Res. 2003;288(1):189–97.
Harrell JC, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66(18):9308–15.
Harrell JC, et al. Estrogen insensitivity in a model of estrogen receptor positive breast cancer lymph node metastasis. Cancer Res. 2007;67(21):10582–91.
Kabos P, DW, Elias A, Horwitz KB, Sartorius CA. The chemoresistant population of luminal subtype human breast cancer cells expresses a basal phenotype. San Antonio Breast Cancer Symposium Proceedings, 2008. Abstract presentation.
Farmer P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15(1):68–74.
Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82.
Bao S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–8.
Sharma D, et al. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19(7):1740–51.
Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther. 2007;6(1):64–9.
Cameron EE, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21(1):103–7.
Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst. 2005;97(20):1498–506.
Bovenzi V, Momparler RL. Antineoplastic action of 5-aza-2′-deoxycytidine and histone deacetylase inhibitor and their effect on the expression of retinoic acid receptor beta and estrogen receptor alpha genes in breast carcinoma cells. Cancer Chemother Pharmacol. 2001;48(1):71–6.
Ferguson AT, et al. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995;55(11):2279–83.
Yang X, et al. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60(24):6890–4.
Yang X, et al. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61(19):7025–9.
Fan J, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134(8):883–90.
Zhou Q, Shaw PG, Davidson NE. Inhibition of histone deacetylase suppresses EGF signaling pathways by destabilizing EGFR mRNA in ER-negative human breast cancer cells. Breast Cancer Res Treat, 2008 Aug 6. doi:10.1007/s10549-008-0148-5.
Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006;66(12):5985–8.
Balasenthil S, et al. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279(2):1422–8.
Holm C, et al. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98(10):671–80.
Munster PN, et al. Phase II trial of vorinostat, a histone deacetylase inhibitor to restore the hormone sensitivity to the anti-estrogen tamoxifen in patients with advanced breast cancer having failed prior aromatase inhibitor therapy. J Clin Oncol. 2008;26(May 20 suppl):abstr 3501.
Acknowledgements
Ciara S. O’Brien is a Cancer Research UK Clinical Training Fellow and Sacha Howell is funded by the Christie Hospital NHS Trust Endowments. Gillian Farnie and Robert Clarke are funded by Breast Cancer Campaign grants 2008MaySF01 and 2006MaySF01, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Brien, C.S., Howell, S.J., Farnie, G. et al. Resistance to Endocrine Therapy: Are Breast Cancer Stem Cells the Culprits?. J Mammary Gland Biol Neoplasia 14, 45–54 (2009). https://doi.org/10.1007/s10911-009-9115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-009-9115-y