Abstract
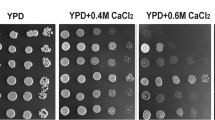
Protein kinase CK2 is essential for the growth of Saccharomyces cerevisiae. Yeast cells that lack the functional genes coding for both the catalytic subunits of protein kinase CK2 can grow only if they are complemented by exogenous cDNAs coding for this subunit. A series of deletion mutants of CK2α from Xenopus laevis was constructed. These mutants that have carboxyl end deletions yield a CK2α product that varies over four orders of magnitude in its capacity to phosphorylate casein in vitro. Complementation of yeast RPG41-1a, a mutant defective in CKA1 and CKA2 genes, with wild-type X. laevis CK2α and with cDNAs coding for truncated CK2α having amino acids 1–328 and 1–327 resulted in cells that grew in gal-minimal media at 30 ∘C as well as the cells harboring the yeast CKA2 gene. However, the growth was significantly diminished when cells were complemented with X. laevis CK2α containing 1–326 amido acids. This mutant has 0.6% of the catalytic activity of the wild-type enzyme. Yeast cells that expressed CK2α 1–324 and 1–323 which have 10-and 100-fold less activity, respectively, were not able to grow. The growth of cells containing the CK2α 1–326 mutant was very sensitive to temperature, and minimal growth was observed at 37 ∘C. This mutant was also more sensitive to UV radiation but was not significantly affected by 0.4 M NaCl.
Similar content being viewed by others
References
Allende JE, Allende CC: Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. Invited Review, FASEB J 9: 313–323, 1995
Guerra B, Issinger OG: Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophor 20: 391–408, 1999
Meggio F, Pinna LA: one thousand and one substrates of protein kinase CK2? FASEB J 17: 349–368, 2003
Ahmed K, Gerber DA, Cochet C: Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12(5): 226–130, 2002
Glover CVC, Bidwai AP, Reed JC: Structure and function of Saccaromyces cerevisiae casein kinase II. Cell Mol Boil Res 40: 481–488, 1994
Padmanabha R, Chen-Wu JLP, Hanna DE, Glover CVC: Isolation, sequencing and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol 10: 4089–4099, 1990
Bidwai AP, Reed JC, Glover CVC: Cloning and disruption of CKB1, the gene encoding the 38-kDa β subunit of Saccharomyces cerevisiae casein kinase II (CKII): deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J Biol Chem 270: 10395–10404, 1995
Reed JC, Bidwai AP, Glover CVC: Cloning and disruption of CKB2, the gene encoding the 32-kDa regulatory B‘-subunit of Saccharomyces cerevisiae casein kinase II. J Biol Chem 269: 18192–18200, 1994
Hanna De, Rethinaswamy A, Glover CVC: casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J Biol Chem 270: 25905–25914, 1995
Tapia J, Jacob G, Allende CC, Allende JE: Role of carboxyl terminus on the catalytic activity of protein kinase CK2α subunit. FEBS Lett 531(2): 363–368, 2002
Gietz D, Jean A, Woods R, Schiestl R: Improved method for high efficient transformation of intact yeast cells. Nucleic Acids Res 20: 1425, 1992
Boeke JD, Trueheart J, Natsoulis G, Fink GR: 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154: 164–175, 1987
Zwietering MH, Jongenburger I, Rombouts FM, van’t Riet K: Modeling of the bacterial growth curve. Appl Environ Microbiol 56: 1875–1881, 1990
Guerra B, Niefind K, Ermakowa I, Issinger OG: Characterization of CK2 holoenzyme variants with regard to crystallization. Mol Cell Biochem 227: 3–11, 2001
Bandhakavi S, Mc Cann RO, Hanna DE, Glover CVC: A positive feed-back loop between protein kinase CKII and Cdc 37 promotes the activity of multiple protein kinases. J Biol Chem 278: 2819–2836, 2003
Miyata Y, Nishida E: CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc 37. Mol Cell Biol 24: 4065–4074, 2004
Author information
Authors and Affiliations
Corresponding author
Additional information
Both authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Hermosilla, G.H., Tapia, J.C. & Allende, J.E. Minimal CK2 activity required for yeast growth. Mol Cell Biochem 274, 39–46 (2005). https://doi.org/10.1007/s11010-005-3112-2
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3112-2