Abstract
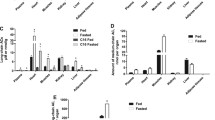
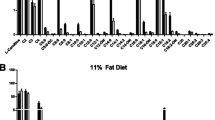
In the heart, a nutritional state (fed or fasted) is characterized by a unique energy metabolism pattern determined by the availability of substrates. Increased availability of acylcarnitines has been associated with decreased glucose utilization; however, the effects of long-chain acylcarnitines on glucose metabolism have not been previously studied. We tested how changes in long-chain acylcarnitine content regulate the metabolism of glucose and long-chain fatty acids in cardiac mitochondria in fed and fasted states. We examined the concentrations of metabolic intermediates in plasma and cardiac tissues under fed and fasted states. The effects of substrate availability and their competition for energy production at the mitochondrial level were studied in isolated rat cardiac mitochondria. The availability of long-chain acylcarnitines in plasma reflected their content in cardiac tissue in the fed and fasted states, and acylcarnitine content in the heart was fivefold higher in fasted state compared to the fed state. In substrate competition experiments, pyruvate and fatty acid metabolites effectively competed for the energy production pathway; however, only the physiological content of acylcarnitine significantly reduced pyruvate and lactate oxidation in mitochondria. The increased availability of long-chain acylcarnitine significantly reduced glucose utilization in isolated rat heart model and in vivo. Our results demonstrate that changes in long-chain acylcarnitine contents could orchestrate the interplay between the metabolism of pyruvate–lactate and long-chain fatty acids, and thus determine the pattern of energy metabolism in cardiac mitochondria.






Similar content being viewed by others
References
Larsen TS, Aasum E (2008) Metabolic (in)flexibility of the diabetic heart. Cardiovasc Drugs Ther 22:91–95
Hue L, Taegtmeyer H (2009) The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297:E578–E591
Liepinsh E, Makrecka M, Kuka J, Makarova E, Vilskersts R, Cirule H, Sevostjanovs E, Grinberga S, Pugovics O, Dambrova M (2014) The heart is better protected against myocardial infarction in the fed state compared to the fasted state. Metabolism 63:127–136
Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283
van de Weijer T, Sparks LM, Phielix E, Meex RC, van Herpen NA, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB (2013) Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One 8:e51648
Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE (1999) Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96:1129–1134
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62:1–8
Dobrzyn P, Pyrkowska A, Jazurek M, Dobrzyn A (2012) Increased availability of endogenous and dietary oleic acid contributes to the upregulation of cardiac fatty acid oxidation. Mitochondrion 12:132–137
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56
Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP (2010) Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18:1695–1700
Ramos-Roman MA, Sweetman L, Valdez MJ, Parks EJ (2012) Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism 61:202–212
van den Broek NM, Ciapaite J, De Feyter HM, Houten SM, Wanders RJ, Jeneson JA, Nicolay K, Prompers JJ (2010) Increased mitochondrial content rescues in vivo muscle oxidative capacity in long-term high-fat-diet-fed rats. FASEB J 24:1354–1364
Hansford RG (1977) Studies on inactivation of pyruvate dehydrogenase by palmitoylcarnitine oxidation in isolated rat heart mitochondria. J Biol Chem 252:1552–1560
Ocloo A, Shabalina IG, Nedergaard J, Brand MD (2007) Cold-induced alterations of phospholipid fatty acyl composition in brown adipose tissue mitochondria are independent of uncoupling protein-1. Am J Physiol Regul Integr Comp Physiol 293:R1086–R1093
Liepinsh E, Skapare E, Kuka J, Makrecka M, Cirule H, Vavers E, Sevostjanovs E, Grinberga S, Pugovics O, Dambrova M (2013) Activated peroxisomal fatty acid metabolism improves cardiac recovery in ischemia-reperfusion. Naunyn Schmiedebergs Arch Pharmacol 386:541–550
Blachnio-Zabielska AU, Koutsari C, Jensen MD (2011) Measuring long-chain acyl-coenzyme A concentrations and enrichment using liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Rapid Commun Mass Spectrom 25:2223–2230
IUPAC-IUB (1967) IUPAC-IUB Commission on Biochemical Nomenclature (CBN). The nomenclature of lipids. Eur J Biochem 2:127–131
Borutaite V, Morkuniene R, Brown GC (1999) Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca(2+)-induced inhibition of substrate oxidation. Biochim Biophys Acta 1453:41–48
Kuka J, Vilskersts R, Cirule H, Makrecka M, Pugovics O, Kalvinsh I, Dambrova M, Liepinsh E (2012) The cardioprotective effect of mildronate is diminished after co-treatment with L-carnitine. J Cardiovasc Pharmacol Ther 17:215–222
Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A (2004) A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem 279:36235–36241
Yoshida Y, Holloway GP, Ljubicic V, Hatta H, Spriet LL, Hood DA, Bonen A (2007) Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J Physiol 582:1317–1335
Toleikis A, Majiene D, Trumbeckaite S, Dagys A, Jasaitis A (1996) The effect of collagenase and temperature on mitochondrial respiratory parameters in saponin-skinned cardiac fibers. Biosci Rep 16:513–519
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258
Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, Shelton JM, McAnally J, Bassel-Duby R, Harris RA, Richardson JA, Kliewer SA (2008) Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 294:H936–H943
Sugden MC, Kraus A, Harris RA, Holness MJ (2000) Fibre-type specific modification of the activity and regulation of skeletal muscle pyruvate dehydrogenase kinase (PDK) by prolonged starvation and refeeding is associated with targeted regulation of PDK isoenzyme 4 expression. Biochem J 346(Pt 3):651–657
Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA (1998) Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J 329(Pt 1):197–201
Ashour B, Hansford RG (1983) Effect of fatty acids and ketones on the activity of pyruvate dehydrogenase in skeletal-muscle mitochondria. Biochem J 214:725–736
Smith SJ, Saggerson ED (1979) Regulation of pyruvate dehydrogenase activity in white adipocyte mitochondria by palmitoyl carnitine and citrate. Int J Biochem 10:785–790
Bugger H, Abel ED (2010) Mitochondria in the diabetic heart. Cardiovasc Res 88:229–240
Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, Cury-Boaventura MF, Silveira LR, Curi R, Hirabara SM (2012) Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis 11:30
Viscarra JA, Ortiz RM (2013) Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism 62:889–897
Soeters MR, Sauerwein HP, Duran M, Wanders RJ, Ackermans MT, Fliers E, Houten SM, Serlie MJ (2009) Muscle acylcarnitines during short-term fasting in lean healthy men. Clin Sci (Lond) 116:585–592
Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, Muoio DM, Dohm GL (2010) Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab 95:3400–3410
Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM (2005) Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280:33588–33598
Muoio DM, Neufer PD (2012) Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15:595–605
Tominaga H, Katoh H, Odagiri K, Takeuchi Y, Kawashima H, Saotome M, Urushida T, Satoh H, Hayashi H (2008) Different effects of palmitoyl-l-carnitine and palmitoyl-CoA on mitochondrial function in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295:H105–H112
Acknowledgments
This study was supported by The European Social Fund projects No. 2009/0147/1DP/1.1.2.1.2/09/IPIA/VIAA/009 and No. 2013/0003/1DP/1.1.1.2.0/13/APIA/VIAA/009 and the Latvian National Research Program BIOMEDICINE.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Makrecka, M., Kuka, J., Volska, K. et al. Long-chain acylcarnitine content determines the pattern of energy metabolism in cardiac mitochondria. Mol Cell Biochem 395, 1–10 (2014). https://doi.org/10.1007/s11010-014-2106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2106-3