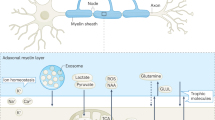
Abstract
Oligodendrocytes are a subtype of glial cells found within the central nervous system (CNS), responsible for the formation and maintenance of specialized myelin membranes which wrap neuronal axons. The development of myelin requires tight coordination for the cell to deliver lipid and protein building blocks to specific myelin segments at the right time. Both internal and external cues control myelination, thus the reception of these signals also requires precise regulation. In late years, a growing body of evidence indicates that oligodendrocytes, like many other cell types, may use extracellular vesicles (EVs) as a medium for transferring information. The field of EV research has expanded rapidly over the past decade, with new contributions that suggest EVs might have direct involvement in communications with neurons and other glial cells to fine tune oligodendroglial function. This functional role of EVs might also be maladaptive, as it has likewise been implicated in the spreading of toxic molecules within the brain during disease. In this review we will discuss the field’s current understanding of extracellular vesicle biology within oligodendrocytes, and their contribution to physiologic and pathologic conditions.

Similar content being viewed by others
References
Chong SY et al (2012) Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA 109:1299–1304. https://doi.org/10.1073/pnas.1113540109
Maier O, Hoekstra D, Baron W (2008) Polarity development in oligodendrocytes: sorting and trafficking of myelin components. J Mol Neurosci 35:35–53. https://doi.org/10.1007/s12031-007-9024-8
Larocca JN, Rodriguez-Gabin AG (2002) Myelin biogenesis: vesicle transport in oligodendrocytes. Neurochem Res 27:1313–1329
Schmitt S, Castelvetri LC, Simons M (1851) Metabolism and functions of lipids in myelin. Biochim Biophys Acta 999–1005:2015. https://doi.org/10.1016/j.bbalip.2014.12.016
Brady ST et al (1999) Formation of compact myelin is required for maturation of the axonal cytoskeleton. J Neurosci 19:7278–7288
Hu Y et al (2004) Synergistic interactions of lipids and myelin basic protein. Proc Natl Acad Sci USA 101:13466–13471. https://doi.org/10.1073/pnas.0405665101
Weimbs T, Stoffel W (1992) Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP. Biochemistry 31:12289–12296. https://doi.org/10.1021/bi00164a002
Dupree JL, Coetzee T, Blight A, Suzuki K, Popko B (1998) Myelin galactolipids are essential for proper node of Ranvier formation in the CNS. J Neurosci 18:1642–1649
Snaidero N et al (2014) Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156:277–290. https://doi.org/10.1016/j.cell.2013.11.044
Gravel M et al (1996) Overexpression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol Cell Neurosci 7:453–466. https://doi.org/10.1006/mcne.1996.0033
Snaidero N et al (2017) Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep 18:314–323. https://doi.org/10.1016/j.celrep.2016.12.053
Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the central nervous system: structure, function, and pathology. Physiol Rev 99:1381–1431. https://doi.org/10.1152/physrev.00031.2018
Rasband MN, Macklin WB (2012) Chapter 10: Myelin structure and biochemistry. In: Brady ST, Siegel GJ, Albers W, Price DL (eds) Basic neurochemistry. Academic Press, Cambridge, pp 180–199
Orentas DM, Miller RH (1996) The origin of spinal cord oligodendrocytes is dependent on local influences from the notochord. Dev Biol 177:43–53. https://doi.org/10.1006/dbio.1996.0143
Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8:a020453. https://doi.org/10.1101/cshperspect.a020453
Barres BA, Raff MC (1999) Axonal control of oligodendrocyte development. J Cell Biol 147:1123–1128. https://doi.org/10.1083/jcb.147.6.1123
Dumas L et al (2015) Multicolor analysis of oligodendrocyte morphology, interactions, and development with Brainbow. Glia 63:699–717. https://doi.org/10.1002/glia.22779
Volpi VG, Touvier T, D’Antonio M (2016) Endoplasmic reticulum protein quality control failure in myelin disorders. Front Mol Neurosci 9:162. https://doi.org/10.3389/fnmol.2016.00162
Rose J et al (2017) Mitochondrial dysfunction in glial cells: implications for neuronal homeostasis and survival. Toxicology 391:109–115. https://doi.org/10.1016/j.tox.2017.06.011
Redmann M, Darley-Usmar V, Zhang J (2016) The Role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis 7:150–162. https://doi.org/10.14336/AD.2015.0820
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927. https://doi.org/10.1152/physrev.2001.81.2.871
Basso M, Bonetto V (2016) Extracellular vesicles and a novel form of communication in the brain. Front Neurosci 10:127. https://doi.org/10.3389/fnins.2016.00127
Willms E et al (2016) Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6:22519. https://doi.org/10.1038/srep22519
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Tricarico C, Clancy J, D’Souza-Schorey C (2017) Biology and biogenesis of shed microvesicles. Small GTPases 8:220–232. https://doi.org/10.1080/21541248.2016.1215283
Trams EG, Lauter CJ, Salem N Jr, Heine U (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645:63–70. https://doi.org/10.1016/0005-2736(81)90512-5
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. https://doi.org/10.1038/nrm.2017.125
Consortium, E.-T. et al (2017) EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14:228–232. https://doi.org/10.1038/nmeth.4185
Lotvall J et al (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for extracellular vesicles. J Extracell Vesicles 3:26913. https://doi.org/10.3402/jev.v3.26913
Witwer KW et al (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.20360
Kim DK et al (2013) EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.20384
Kalra H et al (2012) Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10:e1001450. https://doi.org/10.1371/journal.pbio.1001450
Keerthikumar S et al (2016) ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 428:688–692. https://doi.org/10.1016/j.jmb.2015.09.019
Ostrowski M et al (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30. https://doi.org/10.1038/ncb2000
Kalra H, Drummen GP, Mathivanan S (2016) Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17:170. https://doi.org/10.3390/ijms17020170
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525. https://doi.org/10.1038/nrm2728
Record M, Carayon K, Poirot M, Silvente-Poirot S (1841) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 108–120:2014. https://doi.org/10.1016/j.bbalip.2013.10.004
Colombo M et al (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126:5553–5565. https://doi.org/10.1242/jcs.128868
Trajkovic K et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. https://doi.org/10.1126/science.1153124
Aubin I et al (2005) A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet 37:803–805. https://doi.org/10.1038/ng1603
Menck K et al (2017) Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles 6:1378056. https://doi.org/10.1080/20013078.2017.1378056
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19:434–446. https://doi.org/10.1016/j.tcb.2009.06.004
Perez-Hernandez D et al (2013) The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 288:11649–11661. https://doi.org/10.1074/jbc.M112.445304
Frey B, Gaipl US (2011) The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Semin Immunopathol 33:497–516. https://doi.org/10.1007/s00281-010-0228-6
Li B, Antonyak MA, Zhang J, Cerione RA (2012) RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31:4740–4749. https://doi.org/10.1038/onc.2011.636
Segawa K, Suzuki J, Nagata S (2014) Flippases and scramblases in the plasma membrane. Cell Cycle 13:2990–2991. https://doi.org/10.4161/15384101.2014.962865
Feldmann A, Winterstein C, White R, Trotter J, Kramer-Albers EM (2009) Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J Neurosci Res 87:1760–1772. https://doi.org/10.1002/jnr.22020
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. https://doi.org/10.1002/0471143030.cb0322s30
Matsumoto J, Stewart T, Banks WA, Zhang J (2017) The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des 23:6206–6214. https://doi.org/10.2174/1381612823666170913164738
Raab-Traub N, Dittmer DP (2017) Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol 15:559–572. https://doi.org/10.1038/nrmicro.2017.60
Gross JC, Chaudhary V, Bartscherer K, Boutros M (2012) Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14:1036–1045. https://doi.org/10.1038/ncb2574
Vyas N et al (2014) Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep 4:7357. https://doi.org/10.1038/srep07357
Ratajczak J et al (2006) Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20:847–856. https://doi.org/10.1038/sj.leu.2404132
Vella LJ et al (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol 211:582–590. https://doi.org/10.1002/path.2145
Danzer KM et al (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42. https://doi.org/10.1186/1750-1326-7-42
Kong SM et al (2014) Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet 23:2816–2833. https://doi.org/10.1093/hmg/ddu099
Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 35:1792–1800. https://doi.org/10.1016/j.neurobiolaging.2014.02.012
Silverman JM et al (2019) CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294:3744–3759. https://doi.org/10.1074/jbc.RA118.004825
Chiasserini D et al (2014) Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics 106:191–204. https://doi.org/10.1016/j.jprot.2014.04.028
Sproviero D et al (2018) Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis patients. Front Neurosci 12:487. https://doi.org/10.3389/fnins.2018.00487
Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287:43108–43115. https://doi.org/10.1074/jbc.M112.404467
Asai H et al (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18:1584–1593. https://doi.org/10.1038/nn.4132
Alvarez-Erviti L et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Andre F et al (2004) Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol (Baltimore, Md.: 1950) 172:2126–2136. https://doi.org/10.4049/jimmunol.172.4.2126
Lai C, Breakefield X (2012) Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. https://doi.org/10.3389/fphys.2012.00228
Trajkovic K et al (2006) Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J Cell Biol 172:937–948. https://doi.org/10.1083/jcb.200509022
Simons M et al (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J Cell Biol 157:327–336. https://doi.org/10.1083/jcb.200110138
Winterstein C, Trotter J, Kramer-Albers EM (2008) Distinct endocytic recycling of myelin proteins promotes oligodendroglial membrane remodeling. J Cell Sci 121:834–842. https://doi.org/10.1242/jcs.022731
Feldmann A et al (2011) Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J Neurosci 31:5659–5672. https://doi.org/10.1523/JNEUROSCI.6638-10.2011
Mironova YA et al (2016) PI(3,5)P2 biosynthesis regulates oligodendrocyte differentiation by intrinsic and extrinsic mechanisms. eLife. https://doi.org/10.7554/elife.13023
Walker WP, Oehler A, Edinger AL, Wagner KU, Gunn TM (2016) Oligodendroglial deletion of ESCRT-I component TSG101 causes spongiform encephalopathy. Biol Cell 108:324–337. https://doi.org/10.1111/boc.201600014
Meraviglia V et al (2016) SNX27, a protein involved in down syndrome, regulates GPR17 trafficking and oligodendrocyte differentiation. Glia 64:1437–1460. https://doi.org/10.1002/glia.23015
Powell D et al (2014) Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiol Aging 35:1562–1569. https://doi.org/10.1016/j.neurobiolaging.2014.01.137
Pietiainen V et al (2013) NDRG1 functions in LDL receptor trafficking by regulating endosomal recycling and degradation. J Cell Sci 126:3961–3971. https://doi.org/10.1242/jcs.128132
Camargo N et al (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15:e1002605. https://doi.org/10.1371/journal.pbio.1002605
Lee S et al (2012) A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9:917–922. https://doi.org/10.1038/nmeth.2105
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333:1647–1651. https://doi.org/10.1126/science.1206998
Demerens C et al (1996) Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA 93:9887–9892. https://doi.org/10.1073/pnas.93.18.9887
Simons M, Lyons DA (2013) Axonal selection and myelin sheath generation in the central nervous system. Curr Opin Cell Biol 25:512–519. https://doi.org/10.1016/j.ceb.2013.04.007
Fruhbeis C et al (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11:e1001604. https://doi.org/10.1371/journal.pbio.1001604
Morris-Love J et al (2019) JC polyomavirus uses extracellular vesicles to infect target cells. MBio. https://doi.org/10.1128/mbio.00379-19
Mills EA, Mao-Draayer Y (2018) Understanding progressive multifocal leukoencephalopathy risk in multiple sclerosis patients treated with immunomodulatory therapies: a bird’s eye view. Front Immunol 9:138. https://doi.org/10.3389/fimmu.2018.00138
Delpech JC, Herron S, Botros MB, Ikezu T (2019) Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci 42:361–372. https://doi.org/10.1016/j.tins.2019.02.007
Kramer-Albers EM et al (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl 1:1446–1461. https://doi.org/10.1002/prca.200700522
Hsu C et al (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189:223–232. https://doi.org/10.1083/jcb.200911018
Bakhti M, Winter C, Simons M (2011) Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem 286:787–796. https://doi.org/10.1074/jbc.M110.190009
Fitzner D et al (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124:447–458. https://doi.org/10.1242/jcs.074088
Kirby L et al (2019) Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10:3887. https://doi.org/10.1038/s41467-019-11638-3
Raposo G et al (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172. https://doi.org/10.1084/jem.183.3.1161
White AB et al (2009) Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci 29:6068–6077. https://doi.org/10.1523/JNEUROSCI.5597-08.2009
Hawkins-Salsbury JA et al (2013) Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J Lipid Res 54:3303–3311. https://doi.org/10.1194/jlr.M039610
D’Auria L et al (2017) Psychosine enhances the shedding of membrane microvesicles: implications in demyelination in Krabbe’s disease. PLoS ONE 12:e0178103. https://doi.org/10.1371/journal.pone.0178103
Strauss K et al (2010) Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 285:26279–26288. https://doi.org/10.1074/jbc.M110.134775
Shaharabani R, Ram-On M, Talmon Y, Beck R (2018) Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc Natl Acad Sci USA 115:11156–11161. https://doi.org/10.1073/pnas.1804275115
Cui QL et al (2017) Sublethal oligodendrocyte injury: a reversible condition in multiple sclerosis? Ann Neurol 81:811–824. https://doi.org/10.1002/ana.24944
Matute C, Domercq M, Perez-Samartin A, Ransom BR (2013) Protecting white matter from stroke injury. Stroke 44:1204–1211. https://doi.org/10.1161/STROKEAHA.112.658328
Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC (2015) Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol 74:218–232. https://doi.org/10.1097/NEN.0000000000000165
Baer AS et al (2009) Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132:465–481. https://doi.org/10.1093/brain/awn334
Acknowledgements
This work was supported in part by the generous contribution of the Legacy of Angels Foundation, National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01NS065808) and Dr. Ralph and Marian Falk Medical Research Trust to E.R.B. (Catalyst Award).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honour of Professor Vittorio Gallo.
Rights and permissions
About this article
Cite this article
Reiter, C.R., Bongarzone, E.R. The Role of Vesicle Trafficking and Release in Oligodendrocyte Biology. Neurochem Res 45, 620–629 (2020). https://doi.org/10.1007/s11064-019-02913-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02913-2