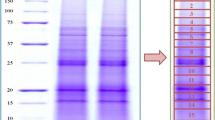
Abstract
Respiratory oxidative phosphorylation represents a central functionality in plant metabolism, but the subunit composition of the respiratory complexes in plants is still being defined. Most notably, complex II (succinate dehydrogenase) and complex IV (cytochrome c oxidase) are the least defined in plant mitochondria. Using Arabidopsis mitochondrial samples and 2D Blue-native/SDS-PAGE, we have separated complex II and IV from each other and displayed their individual subunits for analysis by tandem mass spectrometry and Edman sequencing. Complex II can be discretely separated from other complexes on Blue-native gels and consists of eight protein bands. It contains the four classical SDH subunits as well as four subunits unknown in mitochondria from other eukaryotes. Five of these proteins have previously been identified, while three are newly identified in this study. Complex IV consists of 9–10 protein bands, however, it is more diffuse in Blue-native gels and co-migrates in part with the translocase of the outer membrane (TOM) complex. Differential analysis of TOM and complex IV reveals that complex IV probably contains eight subunits with similarity to known complex IV subunits from other eukaryotes and a further six putative subunits which all represent proteins of unknown function in Arabidopsis. Comparison of the Arabidopsis data with Blue-native/SDS-PAGE separation of potato and bean mitochondria confirmed the protein band complexity of these two respiratory complexes in plants. Two-dimensional Blue-native/Blue-native PAGE, using digitonin followed by dodecylmaltoside in successive dimensions, separated a diffusely staining complex containing both TOM and complex IV. This suggests that the very similar mass of these complexes will likely prevent high purity separations based on size. The documented roles of several of the putative complex IV subunits in hypoxia response and ozone stress, and similarity between new complex II subunits and recently identified plant specific subunits of complex I, suggest novel biological insights can be gained from respiratory complex composition analysis.
Similar content being viewed by others
References
Braun, H.P. and Schmitz, U.K. 1992. Affinity purification of cytochrome c reductase from plant mitochondria. Eur. J. Biochem. 208: 761–767.
Braun, H.P. and Schmitz, U.K. 1995. The bifunctional cytochrome c reductase/processing peptidase complex from plant mitochondria. J. Bioenerg. Biomembr. 27: 423–436.
Braun, H.P., Emmermann, M., Kruft, V. and Schmitz, U.K. 1992a. The general mitochondrial processing peptidase from potato is an integral part of cytochrome c reductase of the respiratory chain. EMBO J. 11: 3219–3227.
Braun, H.P., Emmermann, M., Kruft, V. and Schmitz, U.K. 1992b. Cytochrome c1 from potato: a protein with a presequence for targeting to the mitochondrial intermembrane space. Mol. Gen. Genet. 231: 217–225.
Burger, G., Lang, B.F., Braun, H.P. and Marx, S. 2003. The enigmatic mitochondrial ORF ymf39 codes for ATP synthase chain b. Nucleic Acids Res. 31: 2353–2360.
Burke, J.J., Siedow, J.N. and Moreland, D.E. 1982. Succinate dehydrogenase. A partial purification from mung bean hypocotyls and soybean cotyledons. Plant Physiol. 70: 1577–1581.
Capaldi, R.A. 1990. Structure and function of cytochrome c oxidase. Ann. Rev. Biochem. 59: 569–596.
Combettes, B. and Grienenberger, J.M. 1999. Analysis of wheat mitochondrial complex I purified by a one-step immunoaf-finity chromatography. Biochemie 81: 645–653.
Covello, P.S. and Gray, M.W. 1989. RNA editing in plant mitochondria. Nature 341: 662–666.
Dagsgaard, C., Taylor, L.E., O'Brien, K.M. and Poyton, R.O. 2001. Effects of anoxia and the mitochondrion on expression of aerobic nuclear COX genes in yeast. J. Biol. Chem. 276: 7593–7601.
Eriksson, A.C., Sjöling, S. and Glaser, E. 1994. The ubiquinol cytochrome c oxidoreductase complex of spinach leaf mitochondria is involved in both respiration and protein processing. Biochim. Biophys. Acta 1186: 221–231.
Eubel, H., Jänsch, L. and Braun, H.P. 2003. New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol. 133: 274–286.
Eubel, H., Heinemeyer, J. and Braun, H.P. 2004. Identification and characterization of respirasomes in potato mitochondria. Plant Physiol. 134: 1450–1459.
Fearnley, I.M., Carroll, J., Shannon, R.J., Runswick, M.J., Walker, J.E. and Hirst, J. 2001. GRIM-19, a cell death regulatory gene product, is a subunit of the bovine itochondrial NADH: ubiquinone oxidoreductase (complexI). J. Biol. Chem. 276: 38345–38348.
Figueroa, P., Léon, G., Elorza, A., Holuigue, L. and Jordana, X. 2001. Three different genes encode the iron-sulfur subunit of succinate dehydrogenase in Arabidopsis thaliana. Plant Mol. Biol. 46: 241–250.
Figueroa, P., Léon, G., Elorza, A., Holuigue, L., Araya, A. and Jordana, X. 2002. The four subunits of the mitochondrial respiratory complex II are encoded by the multiple nuclear genes and targeted to mitochondria in Arabidopsis thaliana. Plant Mol. Biol. 50: 725–734.
Fox, T.D. and Leaver, C.J. 1981. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell 26: 315–323.
Gracey, A.Y., Troll, J.V. and Somero, G.N. 2001. Hypoxiainduced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc. Natl. Acad. Sci. U.S.A 98: 1993–1998.
Gualberto, J.M., Lamattina, L., Bonnard, G., Weil, J.H. and Grienenberger, J.M. 1989. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature 341: 660–662.
Hamasur, B. and Glaser, E. 1992. Plant mitochondrial F0F1 ATP synthase. Identification of the individual subunits and properties of the purified spinach leaf mitochondrial ATP synthase. Eur. J. Biochem. 205: 409–416.
Hattori, T. and Asahi, T. 1982. The presence of two forms of succinate dehydrogenase in sweet potato root mitochondria. Plant Cell Physiol. 23: 515–523.
Hawkesford, M.J., Lidell, A.D. and Leaver, C. 1989. Subunit composition of cytochrome c oxidase in mitochondria of Zea mays. Plant Physiol. 91: 1535–1542.
Heazlewood, J.A., Howell, K.A. and Millar, A.H. 2003a. Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and yeast components coupled to plantspecific subunits. Biochim. Biophys. Acta (Bioenergetics) 1604: 159–169.
Heazlewood, J.L., Whelan, J. and Millar, A.H. 2003b. The products of the mitochondrial ORF25 and ORFB genes are FO components of the plant F1FO ATP synthase. FEBS Lett. 540: 201–205.
Heazlewood, J.L., Tonti-Filippini, J.S., Gout, A.M., Day, D.A., Whelan, J. and Millar, A.H. 2004. Experimental analysis of the Arabidopsis mitochondrial proteome highlights signalling and regulatory components, provides assessment of targeting prediction programs and points to plant specific mitochondrial proteins. Plant Cell 16: 241–256.
Heinemeyer, J., Eubel, H., Wehmhöner, D., Jänsch, L. and Braun, H.P. 2004. Proteomic approach to characterize the 88 supramolecular organization of photosystems in higher plants. Phytochemistry 65: 1683–1692.
Herz, U., Schroder, W., Lidell, A., Leaver, C.J., Brennicke, A., Grohmann, L. 1994. Purification of the NADH: ubiquinone oxidoreductase (complex I) of the respiratory chain from the inner mitochondrial membrane of Solanum tuberosum. J. Biol. Chem. 269: 2263–2269.
Hiatt, A.J. 1961. Preparation and some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 36: 552–557.
Hiesel, R., Schobel, W., Schuster, W. and Brennicke, A. 1987. The cytochrome oxidase subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promotor sequences. EMBO J. 6: 29–34.
Hiesel, R., Wissinger, B., Schuster, W. and Brennicke, A. 1989. RNA editing in plant mitochondria. Science 246: 1632–1634.
Igamberdiev, A.U. and Falaleeva, M.I. 1994. Isolation and characterization of the succinate-dehydrogenase complex from plant mitochondria. Biochemistry (Moscow) 59: 895–900.
Issac, P.G., Jones, V.P. and Leaver, C.J. 1985. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 4: 1617–1623.
Jänsch, L., Kruft, V., Schmitz, U.K. and Braun, H.P. 1996. New insights into the composition, molecular mass and stoichiometry of the protein complexes of plant mitochondria. Plant J. 9: 357–368.
Kadenbach, B. and Merle, P. 1981. On the function of multiple subunits of cytochrome c oxidase from higher eukaryotes. FEBS Lett. 135: 1–11.
Kadowaki, K., Kubo, N., Ozawa, K. and Hirai, A. 1996. Targeting presequence acquisition after mitochondrial gene transfer to the nucleus occurs by duplication of existing targeting signals. EMBO J. 15: 6652–6661.
Kruft, V., Eubel, H., Werhahn, W., Jänsch, L. and Braun, H.P. 2001. Proteomic approach to identify novel mitochondrial functions in Arabidopsis thaliana. Plant Physiol. 127: 1694–1710.
Lemire, B.L. and Oyedotun, K.S. 2002. The Saccharomyces cerevisiae mitochondrial succinate:ubiquinone oxidoreductase. Biochim. Biophys. Acta 1553: 102–116.
Leterme, S. and Boutry, M. 1993. Purification and preliminary characterization of mitochondrial complex I (NADH:ubiquinone reductase) from broad bean (Vicia faba L.). Plant Physiol. 102: 435–443.
Lupold, D.S., Caoile, A.G. and Stern, D.B. 1999. Polyadenylation occurs at multiple sites in maize mitochondrial cox2 mRNA and is independent of editing status. Plant Cell: 1565–1578.
Maeshima, M. and Asahi, T. 1978. Purification and characterization of sweet potato cytochrome c oxidase. Arch. Biochem. Biophys. 187: 423–430.
Matsuoka, M., Maeshima, M. and Asahi, T. 1981. The subunit composition of pea cytochrome c oxidase. J. Biochem. 90: 649–655.
Michalecka, A.M., Svensson, A.S., Johansson, F.I., Agius, S.C., Johanson, U., Brennicke, A., Binder, S. and Rasmusson, A.G. 2003. Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiol.: 642–652.
Millar, A.H., Mittova, V., Kiddle, G., Heazlewood, J.L., Bartoli, C.G., Theodoulou, F.L. and Foyer, C.H. 2003. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 133: 443–447.
Millar, A.H., Sweetlove, L.J., Giegé, P. and Leaver, C.J. 2001. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 127: 1711–1727.
Moller, I.M. 2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 52: 561–591.
Moore, C.S., Cook-Johnson, R.J., Rudhe, C., Whelan, J., Day, D.A., Wiskich, J.T. and Soole, K.L. 2003. Identification of AtNDI1, an internal non-phosphorylating NAD(P)H dehydrogenase in Arabidopsis mitochondria. Plant Physiol. 133: 1968–1978.
Nakagawa, T., Maeshima, M., Muto, H., Kajiura, H., Hattori, H. and Asahi, T. 1987. Separation, amino-terminal sequence and cell-free synthesis of the smallest subunit of sweet potato cytochrome c oxidase. Eur. J. Biochem. 165: 303–307.
Nakagawa, T., Maeshima, M., Nakamura, K. and Ashahi, T. 1990. Molecular cloning of a cDNA for the smallest nuclearencoded subunit of sweet potato cytochrome c oxidase. Eur. J. Biochem. 191: 557–561.
Neuhoff, V., Stamm, R. and Eibl, H. 1985. Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 6: 427–448.
Neuhoff, V., Stamm, R., Pardowitz, I., Arold, N., Ehrhardt, W., Taube, D. 1990. Essential problems in quantification of proteins following colloidal staining with Coomassie Brilliant Blue dyes in polyacrylamide gels, and their solution. Electrophoresis 11: 101–117.
Parisi, G., Perales, M., Fornasari, M.S., Maria, S., Colaneri, A., Gonzalez-Schain, N., Gomez-Casati, D., Zimmermann, S., Brennicke, A., Araya, A. Ferry, J.G., Echave, J. and Zabaleta, E. 2004. Gamma carbonic anhydrases in plant mitochondria. Plant Mol. Biol. in press
Pfeiffer, W.E., Ingle, R.T. and Ferguson-Miller, S. 1990. Structurally unique plant cytochrome c oxidase isolated from wheat germ, a rich source of plant mitochondrial enzymes. Biochemistry 29: 8696–8701.
Rasmusson, A.G., Mendel-Hartvig, J., Moller, I.M., Wiskich, J.T. 1994. Isolation of the rotenone-sensitive NADH-ubiquinone reductase (complex I) from red beet mitochondria. Physiol. Plant 90: 607–615.
Rasmusson, A.G., Svensson, A.S., Knoop, V., Grohmann, L. and Brennicke, A. 1999. Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: two enzymes are present in potato mitochondria. Plant J. 20: 79–87.
Richter, O.M.H. and Ludwig, B. 2003. Cytochrome c oxidase–structure, function and physiology of a redox-driven molecular machine. Rev. Physiol. Biochem. Pharmacol. 147: 47–74.
Sabar, M., Gagliardi, D., Balk, J. and Leaver, C.J. 2003. ORFB is a subunit of F(1)F(O)-ATP synthase: insight into the basis of cytoplasmic male sterility in sunflower. EMBO Rep. 4: 1–6.
Schägger, H. 2001. Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Bio. 65: 231–244.
Schägger, H. and Pfeiffer, K. 2000. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19: 1777–1783.
Sharma, Y.K. and Davis, K.R. 1995. Isolation of a novel Arabidopsis ozone-induced cDNA by differential display. Plant. Mol. Biol. 29: 91–98.
Smith, M.K., Day, D.A. and Whelan, J. 1994. Isolation of a novel soybean gene encoding a mitochondrial ATP synthase subunit. Arch. Biochem. Biophys. 313: 235–240.
Unseld, M., Marienfeld, J.R., Brandt, P. and Brennicke, A. 1997. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366924 nucleotides. Nat. Genet. 15: 57–61.
Vanlerberghe, G.C. and McIntosh, L. 1997. Alternative oxidase: from gene to function. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 48: 703–734.
Werhahn, W., Niemeyer, A., Jänsch, L., Kruft, V., Schmitz, U.K. and Braun, H.P. 2001. Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis thaliana: identification of multiple forms of TOM20. Plant Physiol. 125: 943–954.
Yankovskaya, V., Horsefield, R., Tornroth, S., Luna-Chavez, C., Miyoshi, H., Leger, C., Byrne, B., Cecchini, G. and Iwata, S. 2003. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299: 700–704.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Millar, A.H., Eubel, H., Jänsch, L. et al. Mitochondrial cytochrome c oxidase and succinate dehydrogenase complexes contain plant specific subunits. Plant Mol Biol 56, 77–90 (2004). https://doi.org/10.1007/s11103-004-2316-2
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-2316-2