Abstract
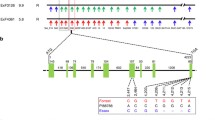
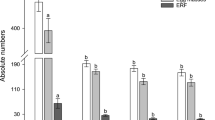
Transcriptional mapping experiments of the major soybean cyst nematode resistance locus, rhg1, identified expression of the vesicular transport machinery component, α soluble NSF attachment protein (α-SNAP), occurring during defense. Sequencing the α-SNAP coding regions from the resistant genotypes G. max [Peking/PI 548402] and G. max [PI 437654] revealed they are identical, but differ from the susceptible G. max [Williams 82/PI 518671] by the presence of several single nucleotide polymorphisms. Using G. max [Williams 82/PI 518671] as a reference, a G → T2,822 transversion in the genomic DNA sequence at a functional splice site of the α-SNAP[Peking/PI 548402] allele produced an additional 17 nucleotides of mRNA sequence that contains an in-frame stop codon caused by a downstream G → A2,832 transition. The G. max [Peking/PI 548402] genotype has cell wall appositions (CWAs), structures identified as forming as part of a defense response by the activity of the vesicular transport machinery. In contrast, the 17 nt α-SNAP[Peking/PI 548402] mRNA motif is not found in G. max [PI 88788] that exhibits defense to H. glycines, but lack CWAs. The α-SNAP[PI 88788] promoter contains sequence elements that are nearly identical to the α-SNAP[Peking/PI 548402] allele, but differs from the G. max [Williams 82/PI 518671] ortholog. Overexpressing the α-SNAP[Peking/PI 548402] allele in the susceptible G. max [Williams 82/PI 518671] genotype suppressed H. glycines infection. The experiments indicate a role for the vesicular transport machinery during infection of soybean by the soybean cyst nematode. However, increased GmEREBP1, PR1, PR2, PR5 gene activity but suppressed PR3 expression accompanied the overexpression of the α-SNAP[Peking/PI 548402] allele prior to infection.






Similar content being viewed by others
References
Abel S, Theologis A (1994) Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J 1994(5):421–427
Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14:145–163
Alkharouf N, Klink VP, Chouikha IB, Beard HS, MacDonald MH, Meyer S, Knap HT, Khan R, Matthews BF (2006) Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224:838–852
An Q, Ehlers K, Kogel KH, van Bel AJ, Hückelhoven R (2006a) Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol 172:563–570
An Q, Hückelhoven R, Kogel KH, van Bel AJ (2006b) Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 8:1009–1019
Antoniw JF, Pierpoint WS (1978) The purification and properties of one of the ‘b” proteins from virus-infected tobacco plants. J Gen Virol 39:343–350
Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, Somerville CR, Thordal-Christensen H (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15:5118–5129
Atkinson HJ, Harris PD (1989) Changes in nematode antigens recognized by monoclonal antibodies during early infections of soya bean with cyst nematode Heterodera glycines. Parasitology 98:479–487
Babcock M, Macleod GT, Leither J, Pallanck L (2004) Genetic analysis of soluble N-ethylmaleimide-sensitive factor attachment protein function in Drosophila reveals positive and negative secretory roles. J Neurosci 24:3964–3973
Bachem CW, Oomen RJF, Kuyt S, Horvath BM, Claassens MM, Vreugdenhil D, Visser RG (2000) Antisense suppression of a potato alpha-SNAP homologue leads to alterations in cellular development and assimilate distribution. Plant Mol Biol 43:473–482
Bancroft I, Morgan C, Fraser F, Higgins J, Wells R, Clissold L, Baker D, Long Y, Meng J, Wang X, Liu S, Trick M (2011) Dissecting the genome of the polyploid crop oilseed rape by transcriptome sequencing. Nat Biotechnol 29:762–766
Barker KR, Koenning SR, Huber SC, Huang JS (1993) Physiological and structural responses of plants to nematode parasitism with Glycine max-Heterodera glycines as a model system. In: Buxon DR, Shibles R, Forsberg RA, Blad BL, Asay KH, Paulsen GM, Wilson RF (eds) International crop science I. Crop Science Society of America, Madison, WI, pp 761–771
Barnard RJ, Morgan A, Burgoyne RD (1996) Domains of alpha-SNAP required for the stimulation of exocytosis and for N-ethylmalemide-sensitive fusion protein (NSF) binding and activation. Mol Biol Cell 7:693–701
Barszczewski M, Chua JJ, Stein A, Winter U, Heintzmann R, Zilly FE, Fasshauer D, Lang T, Jahn R (2008) A novel site of action for alpha-SNAP in the SNARE conformational cycle controlling membrane fusion. Mol Biol Cell 19:776–784
Bekal S, Niblack TL, Lambert KN (2003) A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol Plant-Microbe Interact 16:439–446
Bekal S, Craig JP, Hudson ME, Niblack TL, Domier LL, Lambert KN (2008) Genomic DNA sequence comparison between two inbred soybean cyst nematode biotypes facilitated by massively parallel 454 micro-bead sequencing. Mol Genet Genomics 279:535–543
Bennett MK, Calakos N, Scheller RH (1992) Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257:255–259
Bernard P, Couturier M (1991) The 41 Carboxy-terminal residues of the Mini-F plasmid ccdA protein are sufficient to antagonize the killer activity of the CcdB protein. Mol Gen Genet 226:297–304
Bhattacharyya S, Dey N, Maiti IB (2002) Analysis of cis-sequence of subgenomic transcript promoter from the Figwort mosaic virus and comparison of promoter activity with the cauliflower mosaic virus promoters in monocot and dicot cells. Virus Res 90:47–62
Brucker E, Carlson S, Wright E, Niblack T, Diers B (2005) Rhg1 alleles from soybean PI 437654 and PI 88788 respond differently to isolates of Heterodera glycines in the greenhouse. Theor Appl Genet 111:44–49
Byrd DW Jr, Kirkpatrick T, Barker KR (1983) An improved technique for clearing and staining plant tissue for detection of nematodes. J Nematol 15:142–143
Caldwell BE, Brim CA, Ross JP (1960) Inheritance of resistance of soybeans to the soybean cyst nematode, Heterodera glycines. Agron J 52:635–636
Chai Q, Arndt JW, Dong M, Tepp WH, Johnson EA, Chapman ER, Stevens RC (2006) Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 444:1096–1100
Chen W, Chao G, Singh KB (1996) The promoter of a H2O2-inducible, Arabidopsis glutathione S-transferase gene contains closely linked OBF- and OBP1-binding sites. Plant J 10:955–966
Chicka MC, Hui E, Liu H, Chapman ER (2008) Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat Struct Mol Biol 15:827–835
Clary DO, Griff IC, Rothman JE (1990) SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell 61:709–721
Colgrove AL, Niblack TL (2008) Correlation of female indices from virulence assays on inbred lines and field populations of Heterodera glycines. J Nematol 40:39–45
Collier R, Fuchs B, Walter N, Kevin Lutke W, Taylor CG (2005) Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J 43:449–457
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, Schulze-Lefert P (2003) SNARE-protein mediated disease resistance at the plant cell wall. Nature 425:973–977
Concibido VC, Denny RL, Boutin SR, Hautea R, Orf JH, Young ND (1994) DNA Marker analysis of loci underlying resistance to soybean cyst nematode (Heterodera glycines Ichinohe). Crop Sci 34:240–246
Concibido VC, Denny RL, Lange DA, Orf JH, Young ND (1996) RFLP mapping and marker-assisted selection of soybean cyst nematode resistance in PI 209332. Crop Sci 36:1643–1650
Concibido VC, Lange DA, Denny RL, Orf JH, Young ND (1997) Genome mapping of soybean cyst nematode resistance genes in ‘Peking’, PI 90763, and PI 88788 using DNA markers. Crop Sci 37:258–264
Concibido VC, Diers BW, Arelli PR (2004) A decade of QTL mapping for cyst nematode resistance in soybean. Crop Sci 44:1121–1131
Cregan PB, Mudge J, Fickus EW, Danesh D, Denny R, Young ND (1999a) Two simple sequence repeat markers to select for soybean cyst nematode resistance conditioned by the rhg1 locus. Theor Appl Genet 99:811–818
Cregan PB, Mudge J, Fickus EW, Marek LF, Danesh D, Denny R, Shoemaker RC, Matthews BF, Jarvik T, Young ND (1999b) Targeted isolation of simple sequence repeat markers through the use of bacterial artificial chromosomes. Theor Appl Genet 98:919–928
De Boer JM, Yan Y, Wang X, Smant G, ussey RS, Davis EL (1999) Developmentla expression of secretory β 1, 4-endonucleases in the subventral esophageal glands of Heterodera glycines. Mol Plant Microbe Interact 12:663–669
De Boer JM, Mc Dermott JP, Davis EL, Husses RS, Popeijus H, Smant G, Baum TJ (2002) Cloning of a putative pectate lyase gene expressed in the subventral esophageal glands of Heterodera glycines. J. Nematol 34:9–11
de Ruijter NCA, Verhees J, van Leeuwen W, van der Krol AR (2003) Evaluation and comparison of the GUS, LUC and GFP reporter system for gene expression studies in plants. Plant Biol 5:103–115
Diers BW, Rao-Arelli P (1999) Management of parasitic nematodes of soybean through genetic resistance. In: Kauffman HE (ed) Proceedings of the world soybean research conference, 6th. Chicago, IL. Aug 4–7, 1999. Superior Printing, Champaign, IL, pp 300–306
Doyle JJ, Doyle JL, Brown AH (1999) Origins, colonization, and lineage recombination in a widespread perennial soybean polyploid complex. Proc Natl Acad Sci 96:10741–10745
Edens RM, Anand SC, Bolla RI (1995) Enzymes of the phenylpropanoid pathway in soybean infected with Meloidogyne incognita or Heterodera glycines. J Nematol 27:292–303
Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res 4:388–396
Endo BY (1964) Penetration and development of Heterodera glycines in soybean roots and related and related anatomical changes. Phytopathology 54:79–88
Endo BY (1965) Histological responses of resistant and susceptible soybean varieties, and backcross progeny to entry development of Heterodera glycines. Phytopathology 55:375–381
Endo BY (1991) Ultrastructure of initial responses of susceptible and resistant soybean roots to infection by Heterodera glycines. Revue Nématol 14:73–84
Endo BY, Veech JA (1970) Morphology and histochemistry of soybean roots infected with Heterodera glycines. Phytopathology 60:1493–1498
Epps JM, Hartwig EE (1972) Reaction of soybean varieties and strains to soybean cyst nematode. J Nematol 4:222
Furuta N, Fujita N, Noda T, Yoshimori T, Amano A (2010) Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol Biol Cell 21:1001–1010
Geelen D, Leyman B, Batoko H, Di Sansebastiano Gian-Pietro GP, Moore I, Blatt MR (2002) The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: evidence from competition with its cytosolic domain. Plant Cell 14:387–406
Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC (1994) Synaptotagmin I: a major Ca2 + sensor for transmitter release at a central synapse. Cell 79:717–727
Gipson I, Kim KS, Riggs RD (1971) An ultrastructural study of syncytium development in soybean roots infected with Heterodera glycines. Phytopathology 61:347–353
Goda Y, Stevens CF (1994) Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA 91:12942–12946
Golden AM, Epps JM, Riggs RD, Duclos LA, Fox JA, Bernard RL (1970) Terminology and identity of infraspecific forms of the soybean cyst nematode (Heterodera glycines). Plant Dis Rep 54:544–546
Graham ME, Burgoyne RD (2000) Comparison of cysteine string protein (Csp) and mutant alpha-SNAP overexpression reveals a role for csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J Neurosci 20:1281–1289
Haas JH, Moore LW, Ream W, Manulis S (1995) Universal PCR primers for detection of phytopathogenic Agrobacterium strains. Appl Environ Microbiol 61:2879–2884
Hardham AR, Takemoto D, White RG (2008) Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol 8:63
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H (1995) Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J 14:2317–2325
Hermsmeier D, Mazarei M, Baum TJ (1998) Differential display analysis of the early compatible interaction between soybean and the soybean cyst nematode. Mol Plant Microbe Interact 11:1258–1263
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Hoefle C, Loehrer M, Schaffrath U, Frank M, Schultheiss H, Hückelhoven R (2009) Transgenic suppression of cell death limits penetration success of the soybean rust fungus Phakopsora pachyrhizi into epidermal cells of barley. Phytopathology 99:220–226
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NH, Mattsson O, Jørgensen LB, Jones JD, Mundy J, Petersen M (2009) Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 137:773–783
Holroyd C, Kistner U, Annaert W, Jahn R (1999) Fusion of endosomes involved in synaptic vesicle recycling. Mol Biol Cell 10:3035–3044
Hong K–K, Chakravarti A, Takahashi JS (2004) The gene for soluble N-ethylmaleimide sensitive factor attachment protein α is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci USA 101:1748–1753
Hyten DL, Choi IY, Song Q, Shoemaker RC, Nelson RI, Costa JM, Specht JE, Cregan PB (2010) Highly variable patterns of linkage disequilibrium in multiple soybean populations. Genetics 175:1937–1944
Ibrahim HM, Alkharouf NW, Meyer SL, Aly MA, Gamal El-Din Ael K, Hussein EH, Matthews BF (2011) Post-transcriptional gene silencing of root-knot nematode in transformed soybean roots. Exp Parasitol 127:90–99
Imai A, Hanzawa Y, Komura M, Yamamoto KT, Komeda Y, Takahashi T (2006) The dwarf phenotype of the Arabidopsis acl5 mutant is suppressed by a mutation in an upstream ORF of a bHLH gene. Development 133:3575–3585
Inoue A, Obata K, Akagawa K (1992) Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. J Biol Chem 267:10613–10619
Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, Kihara A, Yoshimori T, Noda T, Ohsumi Y (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell 12:3690–3702
Ithal N, Recknor J, Nettleston D, Hearne L, Maier T, Baum TJ, Mitchum MG (2007) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact 20:293–305
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jenkins WR (1964) A rapid centrifugal flotation technique for separating nematodes from soil. Plant Dis Rep 48:692
Jin R, Rummel A, Binz T, Brunger AT (2006) Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444:1092–1095
Jones MGK (1981) The development and function of plant cells modified by endoparasitic nematodes. In: Zuckerman BM, Rohde RA (eds) Plant Parasitic Nematodes, vol III. Academic Press, New York, pp 255–279
Jones MGK, Northcote DH (1972) Nematode-induced syncytium-a multinucleate transfer cell. J Cell Sci 10:789–809
Kaiser CA, Schekman R (1990) Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61:723–733
Kalde M, Nühse TS, Findlay K, Peck SC (2007) The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci USA 104:11850–11855
Kandoth PK, Ithal N, Recknor J, Maier T, Nettleton D, Baum TJ, Mitchum MG (2011) The Soybean Rhg1 locus for resistance to the soybean cyst nematode Heterodera glycines regulates the expression of a large number of stress- and defense-related genes in degenerating feeding cells. Plant Physiol 155:1960–1975
Kauffmann S, Legrand M, Geoffroy P, Fritig B (1987) Biological function of ‘pathogenesis-related” proteins: four PR proteins of tobacco have 1,3-β-glucanase activity. EMBO J 6:3209–3212
Kauffmann S, Legrand M, Fritig B (1990) Isolation and characterization of six pathogenesis-related (PR) proteins of Samsun NN tobacco. Plant Mol Biol 14:381–390
Kim KS, Riggs RD (1992) Cytopathological reactions of resistant soybean plants to nematode invasion. In: Wrather JA, Riggs RD (eds) Biology and management of the soybean cyst nematode. APS Press, St. Paul, pp 157–168
Kim YH, Riggs RD, Kim KS (1987) Structural changes associated with resistance of soybean to Heterodera glycines. J Nematol 19:177–187
Kim DG, Riggs RD, Mauromoustakos A (1998) Variation in resistance of soybean lines to races of Heterodera glycines. J Nematol 30:184–191
Kim M, Hyten DL, Bent AF, Diers BW (2010) Fine mapping of the SCN resistance locus rhg1-b from PI 88788. Plant Genome 3:81–89
Klink VP, MacDonald M, Alkharouf N, Matthews BF (2005) Laser capture microdissection (LCM) and expression analyses of Glycine max (soybean) syncytium containing root regions formed by the plant pathogen Heterodera glycines (soybean cyst nematode). Plant Mol Biol 59:969–983
Klink VP, Overall CC, Alkharouf N, MacDonald MH, Matthews BF (2007) Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytial cells isolated from incompatible and compatible soybean roots infected by soybean cyst nematode (Heterodera glycines). Planta 226:1389–1409
Klink VP, MacDonald MH, Martins VE, Park S-C, Kim K-H, Baek S-H, Matthews BF (2008) MiniMax, a new diminutive Glycine max variety, with a rapid life cycle, embryogenic potential and transformation capabilities. Plant Cell, Tissue Organ Cult 92:183–195
Klink VP, Hosseini P, Matsye P, Alkharouf N, Matthews BF (2009a) A gene expression analysis of syncytia laser microdissected from the roots of the Glycine max (soybean) genotype PI 548402 (Peking) undergoing a resistant reaction after infection by Heterodera glycines (soybean cyst nematode). Plant Mol Biol 71:525–567
Klink VP, Kim K-H, Martins VE, MacDonald MH, Beard HS, Alkharouf NW, Lee S-K, Park S-C, Matthews BF (2009b) A correlation between host-mediated expression of parasite genes as tandem inverted repeats and abrogation of the formation of female Heterodera glycines cysts during infection of Glycine max. Planta 230:53–71
Klink VP, Hosseini P, MacDonald MH, Alkharouf N, Matthews BF (2009c) Population-specific gene expression in the plant pathogenic nematode Heterodera glycines exists prior to infection and during the onset of a resistant or susceptible reaction in the roots of the Glycine max genotype Peking. BMC-Genomics 10:111
Klink VP, Hosseini P, Matsye P, Alkharouf N, Matthews BF (2010a) Syncytium gene expression in Glycine max [PI 88788] roots undergoing a resistant reaction to the parasitic nematode Heterodera glycines. Plant Physiol Biochem 48:176–193
Klink VP, Overall CC, Alkharouf N, MacDonald MH, Matthews BF (2010b) Microarray detection calls as a means to compare transcripts expressed within syncytial cells isolated from incompatible and compatible soybean (Glycine max) roots infected by the soybean cyst nematode (Heterodera glycines). J Biomed Biotechnol 2010: 491217
Klink VP, Matsye PD, Lawrence GW (2011a). Developmental genomics of the resistant reaction of soybean to the soybean cyst nematode. In: Kumar A, Roy S (eds) Plant tissue culture and applied biotechnology. Aavishkar Publishers, Distributors, India, pp 249–270
Klink VP, Hosseini P, Matsye PD, Alkharouf N, Matthews BF (2011b) Differences in gene expression amplitude overlie a conserved transcriptomic program occurring between the rapid and potent localized resistant reaction at the syncytium of the Glycine max genotype Peking (PI 548402) as compared to the prolonged and potent resistant reaction of PI 88788. Plant Mol Bio 75:141–165
Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, El Kasmi F, Jürgens G, Parker J, Panstruga R, Lipka V, Schulze-Lefert P (2008) Co-option of a default secretory pathway for plant immune responses. Nature 451:835–840
Lai Z, Wang F, Zheng Z, Fan B, Chen Z (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 66:953–968
Lambert KN, Allen KD, Sussex IM (1999) Cloning and characterization of an esophageal-gland specific chorismate mutase from the phytopathogenic nematode Meloidogyne javanica. Mol Plant Microbe Interact 12:328–336
Legrand M, Kauffman S, Geoffroy P, Fritig B (1987) Biological function of pathogenesis-related proteins: four tobacco pathogenesis related proteins are chitinases. Proc Natl Acad Sci USA 84:6750–6754
Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, Bassham DC, Vierstra RD, Parker JE, Bautor J, Molina A, Escudero V, Shindo T, van der Hoorn RA, Gust AA, Nürnberger T (2011) Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 66:818–830
Leroux O, Leroux F, Bagniewska-Zadworna A, Knox JP, Claeys M, Bals S, Viane RL (2011) Ultrastructure and composition of cell wall appositions in the roots of Asplenium (Polypodiales). Micron 42:863–870
Li J, Todd TC, Oakley TR, Lee J, Trick HN (2010) Host derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines. Planta 232:775–785
Li P, Wind JJ, Shi X, Zhang H, Hanson J, Smeekens SC, Teng S (2011a) Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc Natl Acad Sci USA 108:3436–3441
Li Y-H, Qi X-T, Chang R, Qiu L-J (2011) Evaluation and utilization of soybean germplasm for resistance to cyst nematode in China. In: Aleksandra Sudaric (ed) Soybean—molecular aspects of breeding. Intech Publishers, pp 373–396
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Ma Y, Wang W, Liu X, Ma F, Wang P, Chang R, Qiu L (2006) Characteristics of soybean genetic diversity and establishment of applied core collection for Chinese soybean cyst nematode resistance. J Intergrative Biol 48:722–731
Mahalingam R, Wang G, Knap HT (1999) Polygalacturonidase and polygalacturonidase inhibitor protein: gene isolation and transcription in Glycine max-Heterodera glycines interactions. Mol Plant Microbe Interact 12:490–498
Mahalingham R, Skorupska HT (1996) Cytological expression of early response to infection by Heterodera glycines Ichinohe in resistant PI 437654 soybean. Genome 39:986–998
Malhotra V, Orci L, Glick BS, Block MR, Rothman JE (1988) Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell 54:221–227
Martens S, Kozlov MM, McMahon HT (2007) How synaptotagmin promotes membrane fusion. Science 316:1205–1208
Matsye PD, Kumar R, Hosseini P, Jones CM, Alkharouf N, Matthews BF, Klink VP (2011) Mapping cell fate decisions that occur during soybean defense responses. Plant Mol Biol 77:513–528
Matthews B, MacDonald MH, Thai VK, Tucker ML (2003) Molecular characterization of argenine kinase in the soybean cyst nematode (Heterodera glycines). J Nematol 35:252–258
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ (2007) GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis MPMI 20:107–119
McLean MD, Hoover GJ, Bancroft B, Makhmoudova A, Clark SM, Welacky T, Simmonds DH, Shelp BJ (2007) Identification of the full-length Hs1 pro−1 coding sequence and preliminary evaluation of soybean cyst nematode resistance in soybean transformed with Hs1 pro−1 cDNA. Can J Botany 85:437–441
McMahon HT, Südhof TC (1995) Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem 270:2213–2217
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Melito S, Heuberger A, Cook D, Diers B, MacGuidwin A, Bent A (2010) A nematode demographics assay in transgenic roots reveals no significant impacts of the Rhg1 locus LRR-Kinase on soybean cyst nematode resistance. BMC Plant Biol 10:104
Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57:986–999
Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB (2010) Fast vesicle fusion in living cells requires at least three SNARE complexes. Science 330:502–505
Mudge J, Cregan PB, Kenworthy JP, Kenworthy WJ, Orf JH, Young ND (1997) Two microsatellite markers that flank the major soybean cyst nematode resistance locus. Crop Sci 37:1611–1615
Mukherjee S, Kallay L, Brett CL, Rao R (2006) Mutational analysis of the intramembranous H10 loop of yeast Nhx1 reveals a critical role in ion homoeostasis and vesicle trafficking. Biochem J 98:97–105
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nakabeppu Y, Nathans D (1991) A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell 64:751–759
Niblack TL, Arelli PR, Noel GR, Opperman CH, Orf JH, Schmitt DP, Shannon JG, Tylka GL (2002) A revised classification scheme for genetically diverse populations of Heterodera glycines. J Nematol 34:279–288
Niblack TL, Lambert KN, Tylka GL (2006) A model plant pathogen from the kingdom animalia: heterodera glycines, the Soybean Cyst Nematode. Annu Rev Phytopathol 44:283–303
Novick P, Field C, Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205–215
Opperman CH, Bird DMK (1998) The soybean cyst nematode, Heterodera glycines: a genetic model system for the study of plant-parasitic nematodes. Curr Opin Plant Biol 1:1342–1346
Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109:3039–3052
Pajonk S, Kwon C, Clemens N, Panstruga R, Schulze-Lefert P (2008) Activity determinants and functional specialization of Arabidopsis PEN1 syntaxin in innate immunity. J Biol Chem 283:26974–26984
Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, Yoon HW, Lee SH, Chung WS, Lim CO, Lee SY, Hong JC, Cho MJ (2004) Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol 135:2150–2161
Patel S, Dinesh-Kumar SP (2008) Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy 4:20–27
Peter F, Wong SH, Subramaniam VN, Tang BL, Hong W (1998) Alpha-SNAP but not gamma-SNAP is required for ER-Golgi transport after vesicle budding and the Rab1-requiring step but before the EGTA-sensitive step. J Cell Sci 111:2625–2633
Pieterse CMJ, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7:456–464
Rao-Arelli AP, Wilcox JA, Myers O, Gibson PT (1997) Soybean germplasm resistant to Races 1 and 2 of Heterodera glycines. Crop Sci 37:1367–1369
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11:1695–1708
Ren Z, Zheng Z, Chinnusamy V, Zhu J, Cui X, Iida K, Zhu JK (2010) RAS1, a quantitative trait locus for salt tolerance and ABA sensitivity in Arabidopsis. Proc Natl Acad Sci USA 107:5669–5674
Riggs RD, Schmitt DP (1988) Complete characterization of the race scheme for Heterodera glycines. J Nematol 20:392–395
Riggs RD, Schmitt DP (1991) Optimization of the Heterodera glycines race test procedure. J Nematol 23:149–154
Riggs RD, Kim KS, Gipson I (1973) Ultrastructural changes in Peking soybeans infected with Heterodera glycines. Phytopathology 63:76–84
Robinson AF, Inserra RN, Caswell-Chen EP, Vovlas N, Troccoli A (1997) Rotylenchulus species: identification, distribution, host ranges, and crop plant resistance. Nematropica 27:127–180
Rodríguez F, Bustos MA, Zanetti MN, Ruete MC, Mayorga LS, Tomes CN (2011) α-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. PLoS ONE 6:e21925
Ross JP (1958) Host-Parasite relationship of the soybean cyst nematode in resistant soybean roots. Phytopathology 48:578–579
Ross JP, Brim CA (1957) Resistance of soybeans to the soybean cyst nematode as determined by a double-row method. Plant Dis Rep 41:923–924
Sakamoto AN, Lan VT, Puripunyavanich V, Hase Y, Yokota Y, Shikazono N, Nakagawa M, Narumi I, Tanaka A (2009) A UVB-hypersensitive mutant in Arabidopsis thaliana is defective in the DNA damage response. Plant J 60:509–517
Salmon MA, Van Melderen L, Bernard P, Couturier M (1994) The antidote and autoregulatory functions of the F plasmid ccdA protein: a genetic and biochemical survey. Mol Gen Genet 244:530–538
Sanford JC, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–510
Schmelzer E (2002) Cell polarization, a crucial process in fungal defence. Trends Plant Sci 7:411–415
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Shannon JG, Arelli PR, Young LD (2004) Breeding for resistance and tolerance. In: Schmitt DP, Wrather JA, Riggs RD (eds) Biology and management of soybean cyst nematode, 2nd edn. Schmitt & Associates of Marceline, Marceline, MO, pp 155–180
Sheen J, Hwang S, Niwa Y, Kobayashi H, Galbraith DW (1995) Green fluorescent protein as a new vital marker in plant cells. Plant J 8:777–784
Smant GA, Stokkermans JPWG, Yan Y, De Boer JM, Baum TJ, Wang X, Hussey RS, Gommers FJ, Henrissat B, Davis EL, Helder J, Schots A, Bakker J (1998) Endogenous cellulases in animals: isolation of 1,4-endoglucanase genes from two species of plant-parasitic nematodes. PNAS USA 95:4906–4911
Sørensen JB, Matti U, Wei SH, Nehring RB, Voets T, Ashery U, Binz T, Neher E, Rettig J (2002) The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc Natl Acad Sci USA 99:1627–1632
Steeves RM, Todd TC, Essig JS, Trick HN (2006) Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct Plant Biol 33:991–999
Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Strotmeier J, Willjes G, Binz T, Rummel A (2012) Human synaptotagmin-II is not a high affinity receptor for botulinum neurotoxin B and G: increased therapeutic dosage and immunogenicity. FEBS Lett 586:310–313
Swanton E, Bishop N, Sheehan J, High S, Woodman P (2000) Disassembly of membrane-associated NSF 20S complexes is slow relative to vesicle fusion and is Ca(2 +)-independent. J Cell Sci 113:1783–1791
Tepfer D (1984) Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 37:959–967
Trujillo M, Kogel KH, Hückelhoven R (2004) Superoxide and hydrogen peroxide play different roles in the nonhost interaction of barley and wheat with inappropriate formae speciales of Blumeria graminis. Mol Plant Microbe Interact 17:304–312
Tyrrell M, Campanoni P, Sutter JU, Pratelli R, Paneque M, Sokolovski S, Blatt MR (2007) Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J 51:1099–1115
Vaghchhipawala Z, Bassuner R, Clayton K, Lewers K, Shoemaker R, Mackenzie S (2001) Modulations in gene expression and mapping of genes associated with cyst nematode infection of soybean. Mol Plant Microbe Interact 14:42–54
Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN (1996) Naturally occurring dominant negative variants of Stat5. Mol Cell Biol 16:6141–6148
Webb DM, Baltazar BM, Rao-Arelli AP, Schupp J, Clayton K, Keim P, Beavis WD (1995) Genetic mapping of soybean cyst nematode race-3 resistance loci in the soybean PI 437.654. Theor Appl Genet 91:574–581
Weidman PJ, Melançon P, Block MR, Rothman JE (1989) Binding of an N-ethylmaleimide-sensitive fusion protein to Golgi membranes requires both a soluble protein(s) and an integral membrane receptor. J Cell Biol 108:1589–1596
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:33–44
Winter U, Chen X, Fasshauer D (2009) A conserved membrane attachment site in alpha-SNAP facilitates N-ethylmaleimide-sensitive factor (NSF)-driven SNARE complex disassembly. J Biol Chem 284:31817–31826
Wrather JA, Anderson TR, Arsyad DM, Tan Y, Ploper LD, Porta-Puglia A, Ram HH, Yorinori JT (2001) Soybean disease loss estimates for the top ten soybean-producing countries in 1998. Can J Plant Pathol 23:115–121
Xu T, Ashery U, Burgoyne RD, Neher E (1999) Early requirement for alpha-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J 18:3293–3304
Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, Dong Y, Gutenkunst RN, Fang L, Huang L, Li J, He W, Zhang G, Zheng X, Zhang F, Li Y, Yu C, Kristiansen K, Zhang X, Wang J, Wright M, McCouch S, Nielsen R, Wang J, Wang W (2012) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30:105–111
Yanagisawa S, Izui K (1993) Molecular cloning of two DNA-binding proteins of maize that are structurally different but interact with the same sequence motif. J Biol Chem 268:16028–16036
Zhang F, Hinnebusch AG (2011) An upstream ORF with non-AUG start codon is translated in vivo but dispensable for translational control of GCN4 mRNA. Nucleic Acids Res 39:3128–3140
Zhang B, Chen W, Foley RC, Büttner M, Singh KB (1995) Interactions between distinct types of DNA binding proteins enhance binding to ocs element promoter sequences. Plant Cell 7:2241–2252
1000 Genomes Project Consortium (2010) A map of human genome variation from population-scale sequencing. Nature 467:1061–1073
Acknowledgments
VPK is thankful for start-up support provided by Mississippi State University and the Department of Biological Sciences. The authors are thankful for greenhouse space provided by the Department of Biochemistry, Molecular Biology, Entomology and Plant Pathology at Mississippi State University; funds in the forms of a competitive Research Improvement Grant; support from the Mississippi Soybean Promotion Board. The authors are thankful for funds provided by the USDA-ARS and RDA-ARS Virtual Lab (RAVL) program and to the United Soybean Board. Thanks are extended to Suchit Salian, Kim Anderson, Adrienne McMorris and Prateek Chaudhari at Mississippi State University for their assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2012_9932_MOESM1_ESM.xls
Supplemental Table 1. PCR primers used for cloning experiments. Column 1: Construct, the targeted gene; Column 2: Genetic background, the employed genotype; Column 3: Primer, the primer used in the reaction (XLS 34 kb)
11103_2012_9932_MOESM2_ESM.xlsx
Supplemental Table 2. Analysis of promoter sequences of α-SNAP[PI 88788], α-SNAP[Peking/PI 548402] and α-SNAP[Williams 82/PI 518671] (XLSX 124 kb)
11103_2012_9932_MOESM3_ESM.doc
Supplemental Figure 1. Alignment of α-SNAP proteins. G. max (W 82) (G. max [Williams 82/PI 518671]) (Glyma18g02590); G. max (Peking)(G. max [Peking/PI 548402]); A. thaliana (ATG56190); Oryza sativa (rice) (Os0818110); human (NM_003827); C. elegans (NM_072698); yeast (Saccharomyces cerevisiae) (YBL050 W); Drosophila melanogaster (AAF49035) (DOC 32 kb)
11103_2012_9932_MOESM4_ESM.doc
Supplemental Figure 2. Alignment of the promoter sequences of α-SNAP[PI 88788], α-SNAP[Peking/PI 548402] and α-SNAP[Williams 82/PI 518671] (DOC 46 kb)
Rights and permissions
About this article
Cite this article
Matsye, P.D., Lawrence, G.W., Youssef, R.M. et al. The expression of a naturally occurring, truncated allele of an α-SNAP gene suppresses plant parasitic nematode infection. Plant Mol Biol 80, 131–155 (2012). https://doi.org/10.1007/s11103-012-9932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9932-z