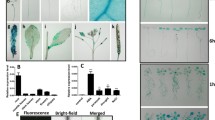
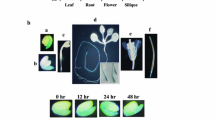
Abstract
Genetic and physiological studies have revealed evidences for multiple signaling pathways by which the plastid exerts retrograde control over photosynthesis-associated-nuclear-genes. In this study we have examined the mechanisms of control of transcription by plastid signals, focusing on transcription factors. We have also further addressed the physical nature of plastid signals and the physiological role, in stress acclimation of this regulatory pathway. ABI4, a master Apetala 2 (AP2)-type transcription factor (TF), is targeted by multiple signalling pathways in plant cells, such as abscisic acid (ABA) signals, sugar signals and plastid signals derived from reactive oxygen species (ROS) and chlorophyll intermediates. ABI4 binds the promoter of target genes to prevent their transcription by competing with other competitive TFs. However, we found that once ABI4 bound the element (CCACGT), it may not be bound by other TFs, therefore making the signalling long-lasting. Downstream of ABI4, CBFA (CCAAT binding factor A) is a subunit of the HAP2/HAP3/HAP5 (Heme activator protein) trimeric transcription complex. CBFA however is a redundant HAP3 subunit. When emergency occurs (such as herbicide treatments or environmental stresses followed by ABA and ROS accumulation), the master transcription factor ABI4 down-regulates some TFs, like CBFA, and then some other TF subunits enter the transcription complex and transcriptional efficiency of stress-responsive genes (including the transcription co-factor CBP) is improved instantaneously. abi4, cbfA and cbp mutants showed weaker drought-tolerance after a herbicide norflurazon treatment, which indicated the physiological role of these key transcription factors.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ABI4:
-
Abscisic acid insensitive 4
- CBFA:
-
CCAAT binding factor A
- CBP:
-
Transcription co-factor CAAT binding protein
- ChIP:
-
Chromatin immunoprecipitation
- GUN1:
-
Genomes uncoupled 1
- HL:
-
High light
- Linc:
-
Lincomycin
- Mg-Proto IX:
-
Mg-protoporphyrin IX
- NF:
-
Norflurazon
- PET:
-
Photosynthetic electron transport chain
- PGE:
-
Plastid gene expression
- PhANGs:
-
Photosynthesis-associated-nuclear-genes
- ROS:
-
Reactive oxygen species
- TF:
-
Transcription factor
References
Ankele E, Kindgren P, Pesquet E, Strand Å (2007) In vivo visualization of Mg-ProtoporphyrinIX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell 19:1964–1979
Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14:2085–2096
Arroyo A, Bossi F, Finkelstein RR, León P (2003) Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol 133:231–242
Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136:3649–3659
Barajas-López Jde D, Blanco NE, Strand Å (2013) Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim Biophys Acta 1833:425–437
Bossi F, Cordoba E, Dupré P, Mendoza MS, Román CS, León P (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59:359–374
Breitenbach J, Zhu CF, Sandmann G (2001) Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors. J Agric Food Chem 49:5270–5272
Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566
Cheng J, He CX, Zhang ZW, Xu F, Zhang DW, Wang X, Yuan S, Lin HH (2011) Plastid signals confer Arabidopsis tolerance to water stress. Z Naturforsch 66c:47–54
Cheng J, Yuan S, Zhang ZW, Zhu F, Tang H, Xu F, Feng H, Xie HF, Xu WL, Lin HH (2012) Plastid-signalling-mediated anthocyanin accumulation in mature Arabidopsis rosettes. Plant Growth Regul 68:223–230
Cottage A, Mott EK, Kempster JA, Gray JC (2010) The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J Exp Bot 61:3773–3786
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Darieva Z, Clancy A, Bulmer R, Williams E, Pic-Taylor A, Morgan BA, Sharrocks AD (2010) A competitive transcription factor binding mechanism determines the timing of late cell cycle-dependent gene expression. Mol Cell 38:29–40
Du SY, Zhang XF, Lu Z, Xin Q, Wu Z, Jiang T, Lu Y, Wang XF, Zhang DP (2012) Roles of the different components of magnesium chelatase in abscisic acid signal transduction. Plant Mol Biol 80:519–537
Enami K, Ozawa T, Motohashi N, Nakamura M, Tanaka K, Hanaoka M (2011) Plastid-to-nucleus retrograde signals are essential for the expression of nuclear starch biosynthesis genes during amyloplast differentiation in tobacco BY-2 cultured cells. Plant Physiol 157:518–530
Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C, Giraud E, Whelan J, David P, Javot H, Brearley C, Hell R, Marin E, Pogson BJ (2011) Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23:3992–4012
Haring M, Offermann S, Danker T, Horst I, Peterhansel C, Stam M (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3:11
He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid dependent growth and brassinosteroid independent cell-death Pathways. Curr Biol 17:1109–1115
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: betaglucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jung HS, Chory J (2010) Signaling between chloroplasts and the nucleus: can a systems biology approach bring clarity to a complex and highly regulated pathway? Plant Physiol 152:453–459
Kakizaki T, Matsumura H, Nakayama K, Che FS, Terauchi R, Inaba T (2009) Coordination of plastid protein import and nuclear gene expression by plastid-to-nucleus retrograde signaling. Plant Physiol 151:1339–1353
Kakizaki T, Yazu F, Nakayama K, Ito-Inaba Y, Inaba T (2012) Plastid signalling under multiple conditions is accompanied by a common defect in RNA editing in plastids. J Exp Bot 63:251–260
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Kauss D, Bischof S, Steiner S, Apel K, Meskauskiene R (2012) FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg++-branch of this pathway. FEBS Lett 586:211–216
Kindgren P, Eriksson MJ, Benedict C, Mohapatra A, Gough SP, Hansson M, Kieselbach T, Strand Å (2011) A novel proteomic approach reveals a role for Mg-protoporphyrin IX in response to oxidative stress. Physiol Plant 141:310–320
Kindgren P, Kremnev D, Blanco NE, de Dios Barajas López J, Fernández AP, Tellgren-Roth C, Small I, Strand Å (2012) The plastid redox insensitive 2 mutant of Arabidopsis is impaired in PEP activity and high light-dependent plastid redox signalling to the nucleus. Plant J 70:279–291
Kleine T, Voigt C, Leister D (2009) Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet 25:185–192
Kobayashi Y, Kanesaki Y, Tanaka A, Kuroiwa H, Kuroiwa T, Tanaka K (2009) Tetrapyrrole signal as a cell-cycle coordinator from organelle to nuclear DNA replication in plant cells. Proc Natl Acad Sci USA 106:803–807
Kobayashi Y, Imamura S, Hanaoka M, Tanaka K (2011) A tetrapyrrole-regulated ubiquitin ligase controls algal nuclear DNA replication. Nat Cell Biol 13:483–487
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Kwong RW, Bui AQ, Lee H, Kwong LW, Fischer RL, Goldberg RB, Harada JJ (2003) LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell 15:5–18
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Lee H, Fischer RL, Goldberg RB, Harada JJ (2003) Arabidopsis LEAFY COTYLEDON1 represents a functionally specialized subunit of the CCAAT binding transcription factor. Proc Natl Acad Sci USA 100:2152–2156
Liu WJ, Chen YE, Tian WJ, Du JB, Zhang ZW, Xu F, Zhang F, Yuan S, Lin HH (2009) Dephosphorylation of photosystem II proteins and phosphorylation of CP29 in barley photosynthetic membranes as a response to water stress. Biochim Biophys Acta 1787:1238–1245
Maity SN, de Crombrugghe B (1998) Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci 23:174–178
Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S (2012) H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem 287:11717–11729
McCormac AC, Terry MJ (2002) Loss of nuclear gene expression during the phytochrome A-mediated far-red block of greening response. Plant Physiol 130:402–414
McCormac AC, Terry MJ (2004) The nuclear genes Lhcb and HEMA1 are differentially sensitive to plastid signals and suggest distinct roles for the GUN1 and GUN5 plastid signalling pathways during de-etiolation. Plant J 40:672–685
Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144:1777–1785
Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A (2008) Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc Natl Acad Sci USA 105:15178–15183
Moulin M, McCormac AC, Terry MJ, Smith AG (2008) The steady state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc Natl Acad Sci USA 105:15184–15189
Müller AH, Hansson M (2009) The barley magnesium chelatase 150-kd subunit is not an abscisic acid receptor. Plant Physiol 150:157–166
Niu X, Helentjaris T, Bate NJ (2002) Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14:2565–2575
Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57:739–759
Oelmüller R, Mohr H (1986) Photooxidative destruction of chloroplast and its consequences for expression of nuclear genes. Planta 167:106–113
Phung TH, Jung HI, Park JH, Kim JG, Back K, Jung S (2011) Porphyrin biosynthesis control under water stress: sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol 157:1746–1764
Ramel F, Mialoundama AS, Havaux M (2013) Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J Exp Bot 64:799–805
Rockwell NC, Su YS, Lagarias JC (2006) Phytochrome structure and signalling mechanisms. Annu Rev Plant Biol 57:837–858
Ruckle ME, Larkin RM (2009) Plastid signals that affect photomorphogenesis in Arabidopsis thaliana are dependent on GENOMES UNCOUPLED 1 and cryptochrome 1. New Phytol 182:367–379
Ruckle ME, DeMarco SM, Larkin RM (2007) Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 19:3944–3960
Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3:1018–1025
Sandmann G, Bramley PM, Böger P (1980) The inhibitory mode of action of the pyridazinone herbicide norflurazon on a cell-free carotenogenic enzyme system. Pestic Biochem Physiol 14:185–191
Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY, Fan RC, Xu YH, Zhang DP (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443:823–826
Sibéril Y, Doireau P, Gantet P (2001) Plant bZIP G-box binding factors. Modular structure and activation mechanisms. Eur J Biochem 268:5655–5666
Söderman EM, Brocard IM, Lynch TJ, Finkelstein RR (2000) Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol 124:1752–1765
Strand Å, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421:79–83
Sun X, Feng P, Xu X, Guo H, Ma J, Chi W, Lin R, Lu C, Zhang L (2011) A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat Commun 2:477
Tsuzuki T, Takahashi K, Inoue S, Okigaki Y, Tomiyama M, Hossain MA, Shimazaki K, Murata Y, Kinoshita T (2011) Mg-chelatase H subunit affects ABA signaling in stomatal guard cells, but is not an ABA receptor in Arabidopsis thaliana. J Plant Res 124:527–538
Voigt C, Oster U, Börnke F, Jahns P, Dietz KJ, Leister D, Kleine T (2010) In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the GUN-type of plastid signalling. Physiol Plant 138:503–519
von Gromoff ED, Alawady A, Meinecke L, Grimm B, Beck CF (2008) Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell 20:552–567
Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306:1183–1185
Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G (2006) CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18:2971–2984
West M, Yee KM, Danao J, Zimmerman JL, Fischer RL, Goldberg RB, Harada JJ (1994) LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell 6:1731–1745
Woodson JD, Chory J (2008) Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet 9:383–395
Woodson JD, Perez-Ruiz JM, Chory J (2011) Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr Biol 21:897–903
Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149:1525–1535
Yang YN, Qi M, Mei CS (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40:909–919
Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141:475–487
Zhang ZW, Yuan S, Xu F, Yang H, Zhang NH, Cheng J, Lin HH (2010a) The plastid hexokinase pHXK: a node of convergence for sugar and plastid signals in Arabidopsis. FEBS Lett 584:3573–3579
Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH (2010b) Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 33:2121–2131
Zhang ZW, Yuan S, Feng H, Xu F, Cheng J, Shang J, Zhang DW, Lin HH (2011a) Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs—new evidence for a signalling role of tetrapyrroles in mature Arabidopsis plants. J Plant Physiol 168:714–721
Zhang ZW, Yuan S, Xu F, Yang H, Chen YE, Yuan M, Xu MY, Xue LW, Xu XC, Lin HH (2011b) Mg-protoporphyrin, haem and sugar signals double cellular total RNAs against herbicide and high-light-derived oxidative stress. Plant Cell Environ 34:1031–1042
Zhang ZW, Cheng J, Xu F, Yuan M, Du JB, Shang J, Wang Y, Du L, Li ZL, Yuan S (2011c) Mammal cells double their total RNAs against diabetes, ischemia reperfusion and malaria-induced oxidative stress. Mol Med 17:533–541
Acknowledgments
We thank Prof. Joanne Chory (The Salk Institute, La Jolla, USA) for gun1-9 seeds. We thank Dr. Xiao-Chao Xu (College of Bioindustry, Chengdu University, China) for technical assistance with ChIP assay and antibody preparation. This work was supported by the National Nature Science Foundation of China (31070210, 91017004 and 30970214), the National Key Basic Research ‘973’ Program of China (2009CB118500), and the Doctoral Foundation of the Ministry of Education (20110181110059).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhong-Wei Zhang and Ling-Yang Feng have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, ZW., Feng, LY., Cheng, J. et al. The roles of two transcription factors, ABI4 and CBFA, in ABA and plastid signalling and stress responses. Plant Mol Biol 83, 445–458 (2013). https://doi.org/10.1007/s11103-013-0102-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0102-8