Abstract
From a chemical point of view methanol is one of the closest analogues of water. Consistent with this idea EPR spectroscopy studies have shown that methanol binds at—or at least very close to—the Mn4O x Ca cluster of photosystem II (PSII). In contrast, Clark-type oxygen rate measurements demonstrate that the O2 evolving activity of PSII is surprisingly unaffected by methanol concentrations of up to 10%. Here we study for the first time in detail the effect of methanol on photosynthetic water-splitting by employing a Joliot-type bare platinum electrode. We demonstrate a linear dependence of the miss parameter for S i state advancement on the methanol concentrations in the range of 0–10% (v/v). This finding is consistent with the idea that methanol binds in PSII with similar affinity as water to one or both substrate binding sites at the Mn4O x Ca cluster. The possibility is discussed that the two substrate water molecules bind at different stages of the cycle, one during the S4 → S0 and the other during the S2 → S3 transition.
Similar content being viewed by others
Introduction
In all oxygen-evolving photosynthetic organisms (cyanobacteria, green algae, and higher plants) water-splitting takes place at the μ-oxo bridged tetra-manganese calcium cluster (Mn4O x Ca, with 4 ≤ x ≤ 8) of photosystem II (PSII). This process is energetically driven by strongly oxidizing equivalents that are generated one at a time following light absorption in the chlorophyll-containing reaction center of PSII (Renger and Holzwarth 2005). In this way the Mn4O x Ca cluster is oxidized stepwise and cycles through five oxidation states that are referred to as S i states (Kok et al. 1970). The index (i = 0,…,4) signifies the number of stored oxidizing equivalents. After the accumulation of four oxidizing equivalents the highly reactive S4 state is reached, which decays under the release of molecular oxygen and rebinding of new substrate water into the S0 state. After dark-adaptation the predominant state is the S1 state, since S0, S2, and S3 are meta-stabile and react to S1 via different pathways (for details see, Hillier and Messinger 2005; Messinger and Renger 2008). This kinetic scheme (Kok cycle) explains the periodicity of four that is observed when dark-adapted thylakoids are illuminated with a train of saturating single turn-over flashes (Joliot et al. 1969).
The S i state turnovers are coupled to miss (α) and double hit (β) probabilities. Therefore the period-four oscillation and damps out within a couple of cycles. The double hit parameter (probability for S i → S i+2 transitions) mostly originates from double turnovers on the acceptor side and its magnitude is dependent on the half-width of the employed flashes. The miss parameter (probability that no S i state advancement occurs) maybe affected by all redox-equilibria and kinetics within PSII and is therefore a very sensitive probe for changes within PSII. The magnitude of α strongly depends on pH and temperature (Messinger and Renger 1994; Messinger et al. 1993), and is also affected by H/D isotope exchange (Christen et al. 1999).
Due to recent progress important details are known about the structure of the Mn4O x Ca cluster and its ligands in the dark-stable S1 state (Ferreira et al. 2004; Loll et al. 2005; Yano et al. 2006) and some information is also available on the number of substrate water molecules and their binding modes in the various S i states (Chu et al. 2000; Evans et al. 2004; Hansson et al. 1986; Hillier and Wydrzynski 2000; Kimura et al. 2005a, b; Messinger et al. 1995; Noguchi et al. 1995; Noguchi and Sugiura 2000, 2002; Radmer and Ollinger 1986; Su et al. 2008; Turconi et al. 1997). However, due to the limited resolution and to radiation damage during the collection of the X-ray diffraction pattern (Yano et al. 2005), the substrate binding sites and the S i state dependent protonation states of the substrate ‘water’ molecules are still a matter of speculation—a fact that currently diminishes the chances of deriving the correct mechanism of photosynthetic water-splitting seriously.
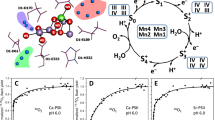
Water is not only the substrate for oxygen evolution, but it is also likely involved in functionally important H-bonding networks that are required for the structural and functional integrity of PSII (Kaminskaya et al. 2003; Noguchi and Sugiura 2002). Therefore, it is not possible to gain direct information on substrate binding by varying the water concentration. Accordingly, isotope labeling, partly in connection with time-resolved methods, has been employed to gather information on water-binding sites and modes at the Mn4O x Ca cluster (for summary see, Hillier and Messinger 2005). An alternative approach is to utilize substrate analogs. Prominent examples for molecules structurally related to water are hydrogen sulfide, methanol, ammonia, hydrogen peroxide, hydroxylamine, and hydrazine (Scheme 1).
Water and methanol differ structurally by the replacement of one H-atom by a CH3-group (Scheme 1). While this exchange affects (i) molecular properties like polarity and H-bonding capacity, and (ii) bulk properties, such as the boiling point, it leaves one end of the molecule structurally identical and increases the overall size of the molecule only to a relatively small extent. These comparatively conservative differences between methanol and water are reflected by the fact that methanol and water are completely mixable at all molar ratios. Because of these properties methanol can be considered to be one of the closest analogs of water.
In previous EPR-based studies it was noticed that most EPR signals of the Mn4O x Ca cluster are affected by the addition of 1–5% methanol. For example, the S3 state parallel mode EPR signal (Matsukawa et al. 1999) and the g = 4.1 perpendicular mode S2 state EPR signal (for review see, Haddy 2007) cannot be observed in presence of methanol or ethanol, while in PSII membrane fragments from higher plants the S0 multiline signal can only be resolved after methanol addition (Messinger et al. 1997a). An ESEEM study employing 2H-labeled methanol clearly showed that methanol (and ethanol) binds at—or at least very close to—the Mn4O x Ca cluster (Force et al. 1998). Consistent with this result it was recently demonstrated by molecular mechanics simulations that an access channel exists that is big enough in diameter to allow methanol (and of cause the smaller water) to diffuse from the lumen to the catalytic Mn4O x Ca cluster (Ho and Styring 2008; see, also Murray and Barber 2007).
In case of ammonia the oxygen of water is exchanged against the isoelectronic NH-group (Scheme 1). Several EPR and FTIR studies suggest that ammonia has one or two binding sites at the Mn4O x Ca cluster (for review see, Debus 1992; Hillier and Messinger 2005). At high enough concentrations ammonia inhibits O2 evolution and alters the S2 state EPR multiline signal (Haddy 2007). The possible competition between ammonia and methanol for binding sites at the water-splitting Mn4O x Ca cluster of photosystem II has been studied by two groups. Based on FTIR measurements Chu and co-workers concluded that methanol is unable to replace ammonia from its binding site at the Mn4O x Ca cluster (Fang et al. 2005), whereas for the reverse exchange experiment Evans et al. found employing electron-spin echo envelope modulation (ESEEM)-spectroscopy for detection that ammonia can displace methanol from its binding site (Evans et al. 2005). These data indicate that ammonia binds more strongly to the Mn4O x Ca cluster than methanol, and that these two water analogs have at least one common binding site.
The question remains whether one or more of these sites are substrate water binding sites. If that were the case one would expect that increasing methanol concentrations should affect the efficiency of oxygen-evolution and possibly also oxidation products of these water-analogs maybe created due to side reactions. The effect of methanol on the oxygen-evolving activity of PSII has so far only been probed by measurements with Clark-type oxygen electrodes. In such experiments suspensions of PSII samples are illuminated for about 1 min with strong white light in the presence of artificial electron acceptors. These kinds of measurements have been performed by several groups and consistently did not reveal any inhibitory effects of methanol at concentrations of up to 5–10% (v/v) (Bernat et al. 2002; Deak et al. 1999; Force et al. 1998). This finding raises the question as to whether or not methanol really acts as substrate analog in PSII. However, one other study (Frasch et al. 1988) reports that methanol can be oxidized by the Mn4O x Ca cluster in the presence of H2O2—a result consistent with methanol binding at a substrate water-binding site.
A third group of molecules structurally related to water is formed by hydrogen peroxide, hydroxylamine, and hydrazine (Scheme 1, lower row). These molecules are, however, not isoelectronic to water. Instead, they are redox-active and known to reduce stepwise the Mn4O x Ca cluster (see, for example Frasch and Mei 1987; Mano et al. 1987; Messinger et al. 1991). While it is still possible that these molecules react via binding to a substrate water-binding site at the Mn4O x Ca cluster, hydrogen peroxide, hydrazine, and hydroxylamine do not appear to bind for long periods of time at the Mn4O x Ca cluster. Hydrogen sulfide has been reported to react in a similar fashion with PSII as hydroxylamine (Sivaraja et al. 1988).
In this study we employ flash-induced oxygen-evolution measurements to probe in detail the effects of small (0–10%, v/v) concentrations of methanol on the efficiency of photosynthetic water-splitting.
Materials and methods
Sample preparation
Spinach thylakoids were isolated from leaves of market spinach as described previously (Winget et al. 1965) with slight modifications outlined in Messinger and Renger (1993). After the final isolation step, thylakoids were frozen as small aliquots in liquid nitrogen and then stored at −80°C until used. These samples were manipulated as described below to populate the reduced or/and oxidized form of tyrosine D (YD and YD ox, respectively). Before the measurements of flash-induced oxygen-evolution patterns (FIOPs) were performed, the thylakoids were thawed in the dark on ice and diluted to [Chl] = 1 mg/ml with medium A (0.4 M sucrose, 15 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, and 50 mM MES/NaOH at pH 6.0). Methanol was added to give final concentrations of 0, 3, 5, 7, and 10% (v/v) about 5 min before the samples were applied onto the Joliot-type electrode (see below).
PSII membrane fragments of ‘BBY’ type were prepared from fresh thylakoid membranes according to the method of Berthold et al. (1981) with minor modifications (Ono and Inoue 1983).
Preparation of ‘S1YD’- and ‘S1YD ox’-thylakoids
-
(i)
S 1 Y D thylakoids contain a high percentage (≥80%) of the reduced form of tyrosine D (YD). These preparations were obtained by long-term (several months) dark-storage at −80°C (Messinger and Renger 1990, 1993; Vass et al. 1990b).
-
(ii)
S 1 Y D ox thylakoids with YD oxidized in about 90% of the centers were obtained by excitation of S1YD samples (pH 6.0; 20°C) with one saturating flash and subsequent 5 min dark-incubation (Messinger and Renger 1990).
Measurements of flash-induced O2-evolution patterns (FIOPs)
FIOPs were obtained in the absence of exogenous electron acceptors with an unmodulated home-built Joliot-type (bare platinum) electrode (Joliot 1972; Messinger 1993) at 20°C (±0.3°C). Sample aliquots of 10 μl were transferred to the electrode in very dim green light. Prior to starting the measurements thylakoids were kept for 3 min on the electrode at given temperature to ensure complete sedimentation and temperature equilibration. The pH of the flow buffer, which also contained the indicated methanol concentrations, was adjusted to the value of 6.0. The polarization voltage of −750 mV was switched on 40 s before flash excitation. Saturating flashes at a frequency of 2 Hz were provided through a polished plexi-glass rod by a xenon flash lamp (Perkin Elmer, LS-1130-4). The amplified amperometric signals were recorded with a personal computer at a sampling rate of 3 ms/point.
S2/S3 lifetime measurements
The S2 and S3 state lifetimes were measured with the Joliot-type electrode. Dark-adapted S1YD-tylakoids were excited with one (S2 state formation) or two (S3 state formation) pre-flash(es). After various dark times, t d (0.5 to 120 s) the O2-yields induced by flash train were recorded and deconvoluted into S i state populations (Isgandarova et al. 2003; Shevela et al. 2006, 2007).
Analysis of FIOPs
The first 16 flashes of each FIOP were analyzed within the framework of different extended Kok models using Excel spreadsheet programs (Kebekus et al. 1995; Messinger et al. 1991, 1997b). All fit programs are based on the formulas:
and
where, S n−1 and S n are vectors of the S i state populations before and after nth flash of the train, K is the matrix containing the Kok parameters α (miss probability) and β (double hit probability), d is an activity parameter that compensates for changes in the number of active PSII centers during the flash train, Y fit n is the oxygen yield due to the nth flash, and [S3] n−1 and [S2] n−1 are the normalized S3 and S2 state populations immediately before the nth flash, respectively. In the most commonly used fit procedure, misses and double hits are assumed to be S i state independent and the S vectors contain only redox states from S0 to S3, i.e.
where, γ is the single hit probability (γ = 1 − α − β).The computer program minimizes the expression
where, \( {\text{Y}}_{n}^{ \exp } \) is the relative O2 yield of the nth flash and F equals the number of analyzed flashes. The normalization is given by
The fit quality is calculated according to Eq. 6:
where, P is the number of free parameters used in the fit. In a more advanced approach, the fit program also includes (i) the reduced S−1 state and (ii) S i state dependent misses (Isgandarova et al. 2003; Messinger et al. 1997b; Shevela et al. 2006). This extended Kok model is summarized in Eq. 7:
where, for example, γ1,n = 1 − α1 − β n is the S i state and flash number (n) dependent single-hit probability. In case of S i state dependent misses, for the sake of simplicity, the α-values for the transitions S0 → S1 and S1 → S2 were fixed to 0 (α1 = α2 = 0) since they are assumed to be insignificantly small. The α-values of S2 → S3 and S3 → S0 were set as free running parameters, but fixed to be equal, i.e., α2 = α3 (Isgandarova et al. 2003; Shevela et al. 2006). For β n it is assumed that the double hit of the first flash (β1) is greater or equal to the value of β in the remaining flashes, which are assumed to then cause identical double hits; i.e., β1 ≥ β2 = β3…. Accordingly, the value of Y n fit was calculated using Eq. 8:
The S i state lifetime data were analyzed by further extending the Kok model by also taking into account the fast reduction of S2 and S3 by YD that can occur during the 500 ms dark-times between flashes of a flash train (Messinger and Renger 1993; Vass et al. 1990a). The required first order rate constants (k f2 , k f3 ) and the percentage of YD in the samples were determined from lifetime measurements in an iterative process that is described in detail in (Isgandarova et al. 2003).
Rate measurements of photosynthetic O2 evolution
The oxygen-evolving activity of PSII membrane fragments was measured with a Clark-type oxygen electrode (Hansatech) at 25°C under continuous actinic illumination (250 W halogen lamp) at the saturating intensity through a Schott GG 455 (2 mm) and Schott KG 3 (2 mm) filters, 8-cm water filter, and 2-cm CuSO4 solution (5%) as a heat filter. Measurements were done at 20 μg Chl/ml in 1-ml chamber filled with buffer A containing the designated MeOH concentrations, and 200 μM PPBQ and 500 μM K3[Fe(CN)6] as artificial electron acceptors. The electrode was calibrated using air-saturated water.
Results
Previous reports showed that methanol concentrations up to 10% (v/v; 2.5 M) do not affect the oxygen-evolution rates (Bernat et al. 2002; Deak et al. 1999; Force et al. 1998), while at 20% (v/v; 5 M) a small, reversible decline was reported (Force et al. 1998). The data in Fig. 1 confirms this finding for PSII membrane (BBY) preparations under our experimental conditions.
Dependence of oxygen-evolving activity on concentrations of methanol in BBY samples as measured by Clark-type electrode after 2-min incubation of the samples in medium A with different concentrations of methanol at pH 6.0 and 25°C under continuous actinic illumination. The assays were performed in the presence of 200 μM PPBQ and 500 μM K3[Fe(CN)6] as electron acceptors. The rate of oxygen evolution of control samples (0% of methanol) was 440 ± 30 μmol O2 (mg Chl)−1 h−1
The top row of Fig. 2 displays original FIOPs obtained with dark-adapted S1YD ox thylakoids (spinach) that contain none (A), 3% (B), or 10% (C) methanol. The control sample without methanol (A) exhibits a pronounced period-four oscillation with a maximum at the 3rd, 7th, 11th, and 15th flash. Inspection of this data readily reveals that with increasing methanol concentration this oscillation becomes less pronounced. These raw data also show that the overall oxygen yields (steady state yields) of the thylakoids decrease only slightly at higher methanol concentrations.
Normalized FIOPs of spinach thylakoids (S1YD ox) measured before (a), after an addition of 3% (b), and 10% (c) of methanol on ice at pH 6.0. Methanol was added to the samples about 3–5 min before FIOP measurements. The measurements were performed without removal of MeOH from the samples. Solid lines correspond to fits A of Table 1, while symbols show experimental values of FIOPs obtained. Broken gray lines with open symbols in (b) and (c) represent FIOPs prior to the addition of MeOH (the same as in a). The inserts show the original, unnormalized FIOPs that were recorded with a flash-frequency of 2 Hz at pH 6.0 and 20°C. No exogenous electron acceptors were added. Normalization of the FIOPs was performed by dividing each flash-induced O2-yield by the O2-yield induced by 3rd flash in ‘control’ sample (a)
To quantify the increased damping caused by the presence of methanol the FIOPs were analyzed by various extended Kok models as described in “Materials and methods.” The obtained fit parameters are listed in Table 1. The fits assuming equal miss probabilities (A1, A2, and A3 in Table 1) are visualized for each condition as solid line in the lower part of Fig. 2 (for comparison the dashed line shows pattern A). Although this fit approach (A1–A3, Table 1) comprises several simplifying assumptions (see “Materials and methods”) it still allows a quite good description of the data (compare solid lines with symbols). While the double hit parameter is hardly affected by methanol (see also Fig. 3b), the miss parameter increases significantly from 9.7% in the control to 19.4% at a methanol concentration of 10%. Figure 3a (closed symbols) includes the results of several intermediate concentrations and shows that the increase in the miss parameter is practically linearly related to the methanol concentration. Importantly, the open symbols in Fig. 3a show that the increased miss probability can be practically fully reversed to normal values by washing the samples in methanol free buffer A. The enhanced miss probability is therefore not due to for example, an irreversible extraction of pigments or denaturation.
Dependence of the miss (top, a) and double hit (bottom, b) probabilities on the presence of different methanol concentrations (% v/v) in S1YD ox (spinach) thylakoids (closed symbols). Parameters obtained after removal of methanol (by washing the samples twice in methanol free buffer A) from the samples are represented by open symbols. The measurements were recorded at 20°C and pH 6.0. The S i state independent misses (α) and double hits (β) were calculated from FIOPs using the extended Kok model described in “Material and methods.” Symbols and error bars represent the average of 2 or 3 independent measurements. All other conditions are as described in Fig. 1
A significant improvement of the fit quality can be achieved if the initial S1 state population is not fixed to 100% and the states S0 and S−1 are included (fits B1–B3). While in this case the miss parameter is lower, the overall trend remains the same. A similar good fit quality can be reached if the miss parameter is assumed to be zero for the S0 → S1 and S1 → S2 state transitions, and the S2 → S3 and S3 → (S4) → S0 transitions are connected with larger miss parameters (fits C1–C3). For simplicity it is assumed that α23 = α30. Since it is unlikely that the preflashed (S1YD ox) samples contain centers in the S0 and S−1 states, we favor fit approach C over B. For fits C1–C3, the values α23 and α30 are higher than the average miss α in the other two approaches. This is expected since the overall miss factor of the cycle is distributed over only two instead of four S i state transitions (if divided by two a very similar value is obtained as for A and B). Also with fits C1–C3 a linear increase of the miss parameter with the methanol concentration is observed (data not shown in Fig. 3).
The above data were obtained with pre-flashed samples, in which most centers are in the state S1YD ox. Therefore, it is unlikely that fast S2 or S3 state decay can explain the observed increase in the miss parameter. However, it cannot be fully excluded that methanol affects the dark-stability of the YD ox radical, which normally is stable for several hours at the employed conditions. In such a scenario both the amplitude and the rate of fast S2 or S3 decay, which are ascribed to the reactions S2YD → S1YD ox and S3YD → S2YD ox, might be functions of the methanol concentration. To test these possibilities S2 and S3 state lifetime measurements were performed with samples enriched in either YD or YD ox. The results of the S2/3YD measurements show that only small changes in the rates of fast S2/S3 decay can be discerned (Table 2). The experiments performed with the pre-flashed (YD ox) samples confirmed that the rates of fast and slow S2 decay are virtually unchanged in presence of 3% methanol (data not shown in Table 2). Interestingly, they also revealed a small increase of the amplitude of the fast phase from 20% (control) to 36% (3% methanol). Calculations with our fit program show (data not presented) that inclusion of the fast decay into the fitting of the FIOPs only insignificantly reduces the above described differences in the miss parameters of control and methanol containing samples. This is also confirmed basically by the full reversibility of the described methanol effect by washing the samples with methanol-free buffer (see Fig. 3)—a treatment that is unable to re-oxidize YD.
Discussion
In this report we describe a surprisingly strong linear relationship between the methanol concentration in the sample and the miss parameter of photosynthetic water-splitting. This methanol induced increase of α is fully reversible at concentrations of up to at least 10% methanol.
The magnitude of the α-value depends on equilibria of both the donor and the acceptor sides of PSII (Renger and Hanssum 1988; Shinkarev and Wraight 1993; Shinkarev 1996, 2005). The almost complete invariance of the double hit parameter β to methanol addition (see Fig. 3b) reveals that acceptor-side effects are negligible. Therefore, the increase of α is ascribed to donor-side effects. It seems most plausible to assume that methanol acts as an effective competitor of water for its binding site that suppresses S i -state advancement when bound. The results also show that methanol exchanges among different PSII complexes during the flash sequence. We have previously used the FIOPs technique to study the exchange rates of herbicides at the QB-site (Vermaas et al. 1984). The present study reveals an exchange rate of methanol that is fast when compared to the dark-time between the flashes (0.5 s), but slow when compared to the lifetime of YZ ox (order of 100 μs). At 10% (v/v) methanol the molar ratio of methanol to water is about 5% and gives rise to an increase of α from 9.7% in the control to 19.4% (see Fig. 3a). One could expect an increase of α of about 5% provided that functional water and methanol exhibit the same binding affinity to the Mn4O x Ca cluster and if all clusters containing one bound methanol molecule are temporarily blocked in the S i -state advancement. In fact, our data show an increase of about 10% thus suggesting that methanol binds somewhat stronger than water (factor of about 2). The proposal of direct binding of methanol to Mn is consistent with two recent ESEEM studies employing isotopically labeled (CD3–OH) methanol (Åhrling et al. 2006; Force et al. 1998).
While the above interpretation is very tempting, further studies are required to probe these ideas. At this stage of the investigation other specific or unspecific interactions of methanol with PSII cannot be fully ruled out.
An increase of α by about 10% is expected to give rise to a decrease of the average oxygen yield per single turnover flash of a similar extent (Messinger and Renger 1994; Christen et al. 1999). Indeed a small decrease of oxygen yield is observed in the FIOPs displayed in Fig. 2. A similar effect cannot be seen up to 10% (v/v; or 2.5 mM) when measuring the oxygen rate under excitation with saturation continuing with white light (Fig. 1, Force et al. 1998), as in this case the activity of PSII is limited by the turnover at the acceptor side. Even if present, an effect of 10% would be within the error of Clark-type measurements. Therefore, the results of Figs. 1 and 2 are not in contradiction with theoretical expectations.
Recently the first 17O-HYSCORE detection of bound (substrate) water was reported (Su et al. 2008). The results obtained resolved only one type of bound water and the possibility was discussed that the presence of 3% methanol may prevent the observation of the second substrate binding site. On the basis of the data from the present study this scenario can be ruled out, since only about 6% of the centers would have been undetectable due to methanol binding to a substrate site. Therefore, the more likely option is that there exists only one type of 17O-exchangeable substrate water-binding site in the S1 and S2 states that can be detected under the conditions of the 17O-HYSCORE technique. On the other hand, results of time-resolved mass spectrometric H2 16O/H 182 O-substrate water exchange experiments suggest that both substrate water molecules are already bound in the S2 state (Hendry and Wydrzynski 2002). However, the reported rate for the fast exchange in S2 is close to the limit of the time resolution of the experiment in which structurally perturbed samples (two extrinsic proteins were removed) have been employed. Therefore, at present the possibility cannot be fully excluded that the two substrate water molecules bind in the Kok cycle at different redox levels S i of the water oxidizing complex (WOC): the first one during the S4 → S0 transition and the second one may bind during the S2 → S3 transition that is known to be coupled with significant structural changes (Boussac and Rutherford 1988; Boussac et al. 1990; Dau et al. 2001; Guiles et al. 1990; Haumann et al. 2005; Liang et al. 2000; Messinger et al. 1991; Noguchi and Sugiura 2002; Pushkar et al. 2008; Renger and Hanssum 1992; Siegbahn 2008). To decide between both possibilities further advanced EPR, FTIR/Raman, and time-resolved membrane-inlet mass spectrometry measurements will be required.
References
Åhrling KA, Evans MCW, Nugent JHA, Ball RJ, Pace RJ (2006) ESEEM studies of substrate water and small alcohol binding to the oxygen-evolving complex of photosystem II during functional turnover. Biochemistry 45:7069–7082. doi:10.1021/bi052146m
Bernat G, Morvaridi F, Feyziyev Y, Styring S (2002) pH dependence of the four individual transitions in the catalytic S-cycle during photosynthetic oxygen evolution. Biochemistry 41:5830–5843. doi:10.1021/bi011691u
Berthold DA, Babcock GT, Yocum CF (1981) A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett 134:231–234. doi:10.1016/0014-5793(81)80608-4
Boussac A, Rutherford AW (1988) Ca2+ binding to the oxygen evolving enzyme varies with the redox state of the Mn cluster. FEBS Lett 236:432–436. doi:10.1016/0014-5793(88)80071-1
Boussac A, Rutherford AW, Styring S (1990) Interaction of ammonia with the water splitting enzyme of photosystem II. Biochemistry 29:24–32. doi:10.1021/bi00453a003
Christen G, Seeliger A, Renger G (1999) P680+ reduction kinetics and redox transition probability of the water oxidizing complex as a function of pH and H/D isotope exchange in spinach thylakoids. Biochemistry 38:6082–6092. doi:10.1021/bi9827520
Chu H-A, Sackett H, Babcock GT (2000) Identification of a Mn–O–Mn cluster vibrational mode of the oxygen evolving complex in photosystem II by low-frequency FTIR spectroscopy. Biochemistry 39:14371–14376. doi:10.1021/bi001751g
Dau H, Iuzzolino L, Dittmer J (2001) The tetra-manganese complex of photosystem II during its redox cycle: X-ray absorption results and mechanistic implications. Biochim Biophys Acta 1503:24–39. doi:10.1016/S0005-2728(00)00230-9
Deak Z, Peterson S, Geijer P, Ahrling KA, Styring S (1999) Methanol modification of the electron paramagnetic resonance signals from the S0 and S2 states of the water-oxidizing complex of photosystem II. Biochim Biophys Acta 1412:240–249. doi:10.1016/S0005-2728(99)00064-X
Debus RJ (1992) The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta 1102:269–352. doi:10.1016/0005-2728(92)90133-M
Evans MCW, Nugent JHA, Ball RJ, Muhiuddin I, Pace RJ (2004) Evidence for a direct manganese-oxygen ligand in water binding to the S2 state of the photosynthetic water oxidation complex. Biochemistry 43:989–994. doi:10.1021/bi035489y
Evans MCW, Ball RJ, Nugent JHA (2005) Ammonia displaces methanol bound to the water oxidizing complex of photosystem II in the S2 state. FEBS Lett 579:3081–3084. doi:10.1016/j.febslet.2005.04.066
Fang CH, Chiang KA, Hung CH, Chang KJ, Ke SC, Chu HA (2005) Effects of ethylene glycol and methanol on ammonia-induced structural changes of the oxygen-evolving complex in photosystem II. Biochemistry 44:9758–9765. doi:10.1021/bi050030k
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838. doi:10.1126/science.1093087
Force DA, Randall DW, Lorigan GA, Clemens KL, Britt RD (1998) ESEEM studies of alcohol binding to the manganese cluster of the oxygen evolving complex of photosystem II. J Am Chem Soc 120:13321–13333. doi:10.1021/ja982713b
Frasch WD, Mei R (1987) Hydrogen peroxide as an alternate substrate for the oxygen evolving complex. Biochim Biophys Acta 891:8–14. doi:10.1016/0005-2728(87)90077-6
Frasch WD, Mei R, Sanders MA (1988) Oxidation of alcohols catalyzed by the oxygen evolving complex. Biochemistry 27:3715–3719. doi:10.1021/bi00410a029
Guiles RD, Zimmermann J-L, McDermott AE, Yachandra VK, Cole JL, Dexheimer SL et al (1990) The S3 state of photosystem II: differences between the structure of the manganese complex in the S2 and S3 states determined by X-ray absorption spectroscopy. Biochemistry 29:471–485. doi:10.1021/bi00454a023
Haddy A (2007) EPR spectroscopy of the manganese cluster of photosystem II. Photosynth Res 92:357–368. doi:10.1007/s11120-007-9194-9
Hansson Ö, Andréasson L-E, Vänngård T (1986) Oxygen from water is coordinated to manganese in the S2 state of photosystem II. FEBS Lett 195:151–154. doi:10.1016/0014-5793(86)80150-8
Haumann M, Müller C, Liebisch P, Iuzzolino L, Dittmer J, Grabolle M et al (2005) Structural and oxidation state changes of the photosystem II manganese complex in four transitions of the water oxidation cycle (S0 → S1, S1 → S2, S2 → S3, and S3, S4 → S0) characterized by X-ray absorption spectroscopy at 20 K and room temperature. Biochemistry 44:1894–1908. doi:10.1021/bi048697e
Hendry G, Wydrzynski T (2002) The two substrate water molecules are already bound to the oxygen evolving complex in the S2 state of photosystem II. Biochemistry 41:13328–13334. doi:10.1021/bi026246t
Hillier W, Messinger J (2005) Mechanism of photosynthetic oxygen production. In: Wydrzynski T, Satoh K (eds) Photosystem II. The light-driven water:plastoquinone oxidoredutase. Advances in photosynthesis and respiration, vol 22. Springer, Dordrecht, pp 567–608
Hillier W, Wydrzynski T (2000) The affinities for the two substrate water binding sites in the O2 evolving complex of photosystem II vary independently during S-state turnover. Biochemistry 39:4399–4405. doi:10.1021/bi992318d
Ho FM, Styring S (2008) Access channels and methanol binding site to the CaMn4 cluster in photosystem II based on solvent accessibility simulations, with implications for substrate water access. Biochim Biophys Acta 1777:140–153. doi:10.1016/j.bbabio.2007.08.009
Isgandarova S, Renger G, Messinger J (2003) Functional differences of photosystem II from Synechococcus elongatus and spinach characterized by flash-induced oxygen evolution patterns. Biochemistry 42:8929–8938. doi:10.1021/bi034744b
Joliot P (1972) Modulated light source use with the oxygen electrode. In: San Pietro A (ed) Photosynthesis and nitrogen fixation. Methods of enzymology, vol 24 B. Academic Press, New York, pp 123–134
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centres photochimiques du systeme II. Photochem Photobiol 10:309–329. doi:10.1111/j.1751-1097.1969.tb05696.x
Kaminskaya O, Renger G, Shuvalov VA (2003) Effect of dehydration on light-induced reactions in photosystem II: photoreactions of cytochrome b559. Biochemistry 42:8119–8132. doi:10.1021/bi020606v
Kebekus U, Messinger J, Renger G (1995) Structural changes in the water oxidizing complex monitored via the pH dependence of the reduction rate of redox state S1 by hydrazine and hydroxylamine in isolated spinach thylakoids. Biochemistry 34:6175–6182. doi:10.1021/bi00018a021
Kimura Y, Ishii A, Yamanari T, Ono TA (2005a) Water-sensitive low-frequency vibrations of reaction intermediates during S-state cycling in photosynthetic water oxidation. Biochemistry 44:7613–7622. doi:10.1021/bi048203d
Kimura Y, Yamanari T, Ishii A, Ono T (2005b) FTIR detection of water-sensitive low-frequency vibrational modes during photosynthetic water oxidation in photosystem II. Plant Cell Physiol 46:S26. doi:10.1093/pcp/pci096
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution. Photochem Photobiol 11:457–476. doi:10.1111/j.1751-1097.1970.tb06017.x
Liang W, Roelofs TA, Cinco RM, Rompel A, Latimer MJ, Yu WO et al (2000) Structural change of the Mn cluster during the S2 to S3 state transition of the oxygen evolving complex of photosystem II. Does it reflect the onset of water/substrate oxidation? Determination by Mn x-ray absorption spectroscopy. J Am Chem Soc 122:3399–3412. doi:10.1021/ja992501u
Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438:1040–1044. doi:10.1038/nature04224
Mano J, Takahashi M-A, Asada K (1987) Oxygen evolution from hydrogen peroxide in photosystem II: flash induced catalytic activity of water oxidizing photosystem II membranes. Biochemistry 26:2495–2501. doi:10.1021/bi00383a014
Matsukawa T, Mino H, Yoneda D, Kawamori A (1999) Dual-mode EPR study of new signals from the S3-state of oxygen-evolving complex in photosystem II. Biochemistry 38:4072–4077. doi:10.1021/bi9818570
Messinger J (1993) Untersuchungen über die reaktiven Eigenschaften der verschiedenen Redoxzustände der Wasseroxidase Höherer Pflanzen. TU Berlin, Berlin
Messinger J, Renger G (1990) The reactivity of hydrazine with PS II strongly depends on the redox state of the water oxidizing system. FEBS Lett 277:141–146. doi:10.1016/0014-5793(90)80829-8
Messinger J, Renger G (1993) Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox States S0, S−1 and S−2 of the water oxidase in isolated spinach thylakoids. Biochemistry 32:9379–9386. doi:10.1021/bi00087a017
Messinger J, Renger G (1994) Analysis of pH-induced modifications of the period four oscillation of the flash induced oxygen evolution reveal distinct structural changes of the photosystem II donor side at characteristic pH values. Biochemistry 33:10896–10905. doi:10.1021/bi00202a008
Messinger J, Renger G (2008) Photosynthetic water-splitting. In: Renger G (ed) Primary processes of photosynthesis—part 2: basic principles and apparatus comprehensive series in photochemical and photobiological sciences. The Royal Society of Chemistry, Cambridge, pp 291–349
Messinger J, Wacker U, Renger G (1991) Unusual low reactivity of the water oxidase in the redox state S3 toward exogenous reductants. Analysis of the NH2OH and NH2NH2 induced modifications of flash induced oxygen evolution in isolated spinach thylakoids. Biochemistry 30:7852–7862. doi:10.1021/bi00245a027
Messinger J, Schröder WP, Renger G (1993) Structure–function relations in photosystem II. Effects of temperature and chaotropic agents on the period four oscillation of flash induced oxygen evolution. Biochemistry 32:7658–7668. doi:10.1021/bi00081a009
Messinger J, Badger M, Wydrzynski T (1995) Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc Natl Acad Sci USA 92:3209–3213. doi:10.1073/pnas.92.8.3209
Messinger J, Nugent JHA, Evans MCW (1997a) Detection of an EPR multiline signal for the S *0 state in photosystem II. Biochemistry 36:11055–11060. doi:10.1021/bi9711285
Messinger J, Seaton G, Wydrzynski T, Wacker U, Renger G (1997b) S−3 state of the water oxidase in photosystem II. Biochemistry 36:6862–6873. doi:10.1021/bi962653r
Murray JW, Barber J (2007) Structural characteristics of channels and pathways in photosystem II including the identification of an oxygen channel. J Struct Biol 159:228–237. doi:10.1016/j.jsb.2007.01.016
Noguchi T, Sugiura M (2000) Structure of an active water molecule in the water oxidizing complex of photosystem II as studied by FTIR spectroscopy. Biochemistry 39:10943–10949. doi:10.1021/bi001040i
Noguchi T, Sugiura M (2002) Flash-induced FTIR difference spectra of the water oxidizing complex in moderately hydrated photosystem II core films: effect of hydration extent on S-state transitions. Biochemistry 41:2322–2330. doi:10.1021/bi011954k
Noguchi T, Ono T-A, Inoue Y (1995) A carboxylate ligand interacting with water in the oxygen evolving center of photosystem II as revealed by Fourier transform infrared spectroscopy. Biochim Biophys Acta 1232:59–66. doi:10.1016/0005-2728(95)00111-3
Ono T-A, Inoue Y (1983) Mn-preserving extraction of 33-, 24- and 16-kDa proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett 164:255–260. doi:10.1016/0014-5793(83)80297-X
Pushkar YL, Yano J, Sauer K, Boussac A, Yachandra VK (2008) Structural changes in the Mn4Ca cluster and the mechanism of photosynthetic water splitting. Proc Natl Acad Sci USA 105:1879–1884. doi:10.1073/pnas.0707092105
Radmer R, Ollinger O (1986) Do the higher oxidation states of the photosynthetic O2 evolving system contain bound water? FEBS Lett 195:285–289. doi:10.1016/0014-5793(86)80178-8
Renger G, Hanssum B (1988) Studies on the deconvolution of flash induced absorption changes into the difference spectra of individual redox steps within the water oxidizing enzyme system. Photosynth Res 16:243–259. doi:10.1007/BF00028843
Renger G, Hanssum B (1992) Studies on the reaction coordinates of the water oxidase in PSII membrane-fragments from spinach. FEBS Lett 299:28–32. doi:10.1016/0014-5793(92)80092-U
Renger G, Holzwarth AR (2005) Primary electron transfer. In: Wydrzynski TJ, Satoh K (eds) Photosystem II. The light-driven water:plastoquinone oxidoreductase. Advances in photosynthesis and respiration, vol 22. Springer, Dordrecht, pp 139–175
Shevela D, Nöring B, Eckert HJ, Messinger J, Renger G (2006) Characterization of the water oxidizing complex of photosystem II of the Chl d-containing cyanobacterium Acaryochloris marina via its reactivity towards endogenous electron donors and acceptors. Phys Chem Chem Phys 8:3460–3466. doi:10.1039/b604389e
Shevela D, Klimov V, Messinger J (2007) Interactions of photosystem II with bicarbonate, formate and acetate. Photosynth Res 94:247–264. doi:10.1007/s11120-007-9200-2
Shinkarev VP (1996) Binary oscillations in the Kok model of oxygen evolution in oxygenic photosynthesis. Photosynth Res 48:411–417. doi:10.1007/BF00029473
Shinkarev VP (2005) Flash-induced oxygen evolution in photosynthesis: simple solution for the extended S-state model that includes misses, double-hits, inactivation, and backward-transitions. Biophys J 88:412–421. doi:10.1529/biophysj.104.050898
Shinkarev V, Wraight CA (1993) Oxygen evolution in photosynthesis: from unicycle to bicycle. Proc Natl Acad Sci USA 90:1834–1838. doi:10.1073/pnas.90.5.1834
Siegbahn PEM (2008) A structure-consistent mechanism for dioxygen formation in photosystem II. Chem Eur J 14:8290–8302. doi:10.1002/chem.200800445
Sivaraja M, Hunziker D, Dismukes GC (1988) The reaction of hydrogen sulfide with the photosynthetic water oxidizing complex and its lack of reaction with the primary electron acceptor in spinach. Biochim Biophys Acta 936:228–235. doi:10.1016/0005-2728(88)90240-X
Su JH, Lubitz W, Messinger J (2008) Probing mode and site of substrate water binding in the oxygen-evolving complex in the S2 state of photosystem II by 17O HYSCORE spectroscopy. J Am Chem Soc 130:786–787. doi:10.1021/ja076620i
Turconi S, MacLachlan DJ, Bratt PJ, Nugent JHA, Evans MCW (1997) Analysis of the interaction of water with the manganese cluster of photosystem II using isotopically labeled water. Biochemistry 36:879–885. doi:10.1021/bi962010b
Vass I, Deak Z, Hideg E (1990a) Charge equilibrium between the water oxidizing complex and the electron donor tyrosine D in photosystem II. Biochim Biophys Acta 1017:63–69. doi:10.1016/0005-2728(90)90179-8
Vass I, Deak Z, Jegerschold C, Styring S (1990b) The accessory electron-donor tyrosine D of photosystem II is slowly reduced in the dark during low-temperature storage of isolated thylakoids. Biochim Biophys Acta 1018:41–46. doi:10.1016/0005-2728(90)90107-F
Vermaas WEJ, Renger G, Dohnt G (1984) The reduction of the oxygen evolving system in chloroplasts by thylakoid components. Biochim Biophys Acta 764:194–202. doi:10.1016/0005-2728(84)90028-8
Winget GD, Izawa S, Good NE (1965) Stoichiometry of photophosphorylation. Biochem Biophys Res Commun 21:438–441. doi:10.1016/0006-291X(65)90401-8
Yano J, Kern J, Irrgang KD, Latimer MJ, Bergmann U, Glatzel P et al (2005) X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc Natl Acad Sci USA 102:12047–12052. doi:10.1073/pnas.0505207102
Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J et al (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314:821–825. doi:10.1126/science.1128186
Acknowledgments
Financial support by the Max-Planck-Gesellschaft (to Johannes Messinger) and Deutsche Forschungsgemeinschaft (to Gernot Renger, Sfb 429 TPA1) is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nöring, B., Shevela, D., Renger, G. et al. Effects of methanol on the S i -state transitions in photosynthetic water-splitting. Photosynth Res 98, 251–260 (2008). https://doi.org/10.1007/s11120-008-9364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-008-9364-4