Abstract
The role of ATP as an extracellular signalling molecule is now well established and evidence is accumulating that ATP and other nucleotides (ADP, UTP and UDP) play important roles in cardiovascular physiology and pathophysiology, acting via P2X (ion channel) and P2Y (G protein-coupled) receptors. In this article we consider the dual role of ATP in regulation of vascular tone, released as a cotransmitter from sympathetic nerves or released in the vascular lumen in response to changes in blood flow and hypoxia. Further, purinergic long-term trophic and inflammatory signalling is described in cell proliferation, differentiation, migration and death in angiogenesis, vascular remodelling, restenosis and atherosclerosis. The effects on haemostasis and cardiac regulation is reviewed. The involvement of ATP in vascular diseases such as thrombosis, hypertension and diabetes will also be discussed, as well as various heart conditions. The purinergic system may be of similar importance as the sympathetic and renin-angiotensin-aldosterone systems in cardiovascular regulation and pathophysiology. The extracellular nucleotides and their cardiovascular P2 receptors are now entering the phase of clinical development.
Similar content being viewed by others
Introduction
Ever since the first proposition of cell surface receptors for nucleotides [1, 2], it has become increasingly clear that, in addition to functioning as an intracellular energy source, the purines and pyrimidines ATP, adenosine diphosphate (ADP), uridine triphosphate (UTP) and uridine diphosphate (UDP) can serve as important extracellular signalling molecules [3, 4] acting on 13 P2X homo- and heteromultimer ionotropic and 8 P2Y metabotropic receptor subtypes [5, 6] (Table 1). To terminate signalling, ectonucleotidases are present in the circulation and on cell surfaces, rapidly degrading extracellular ATP into ADP, AMP and adenosine [7, 8]. Evidence is accumulating suggesting an important role for the purinergic system in cardiovascular regulation [9–15]. It stimulates vasoconstriction and vasodilatation, growth of vascular smooth muscle cells and endothelial cells, angiogenesis, is involved in vascular remodelling, stimulates platelet aggregation, regulates coagulation, inflammation and several aspects of cardiac function. It is involved in blood pressure regulation, development of myocardial infarction, heart failure and xenograft rejection. The physiological effects of the purinergic signalling system are dependent on the release of extracellular nucleotides, the degradation by ectonucleotides, the type of P2 receptors expressed on the cells, their desensitisation rates and their second messengers. P2 receptors have highly specific organ distributions and they can be rapidly up- or downregulated. The purinergic system may be of similar importance as the sympathetic and renin-angiotensin-aldosterone systems in cardiovascular regulation and pathophysiology. The involvement of purinergic signalling in a variety of different cardiovascular clinical conditions has been addressed to a limited extent previously [15–19] and the large number of new discoveries calls for a review to summarise the most important roles of the purinergic system in cardiovascular regulation and disease.
The research field has grown rapidly since the term P2 receptor was coined [2]. In preparation of this review more than 2,700 references on P2 receptors and cardiovascular regulation were found in PubMed. Among the 15 receptor subtypes, one is the target for one of the most widely used medical drugs—the platelet inhibitor clopidogrel (Plavix). As will be reviewed here, several of the other 15 receptors are promising drug targets to prevent cardiovascular disease.
Regulation of vascular tone
Vasoconstriction produced by ATP released as a cotransmitter with noradrenaline (NA) from perivascular sympathetic nerves was recognised early [20, 21]. However, following the seminal discovery of endothelium-dependent vasodilatation by Furchgott in the early 1980s, it was shown that ATP acted on endothelial cells to release endothelial derived relaxing factor [later shown to be largely nitric oxide (NO)] resulting in vasodilatation [22] and dual purinergic neural and endothelial control of vascular tone established [23, 24].
Neuronal regulation of vascular tone
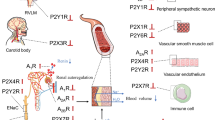
ATP is, together with NA and neuropeptide Y (NPY), a cotransmitter in sympathetic neurons [11, 25, 26], and sensory-motor nerves during “axon reflex” activity release ATP to dilate or constrict vessels [27, 28] (Fig. 1). The contribution of ATP to sympathetic contraction varies between vascular beds [25]. For example, it may mediate up to 50% of the neurogenic vasoconstriction via P2X1 receptors as seen in mesenteric arteries [29], about 70% in rabbit saphenous arteries and 100% in rabbit jejunal artery, while NA acts as a prejunctional modulator [30]. In the renal vasculature both P2X1 and P2Y2 receptors are important for neurogenic contraction. P2X1 receptors mediate contraction in afferent but are absent in the efferent renal arterioles [31, 32]. The result is that extracellular nucleotides selectively influence preglomerular resistance without having an effect on postglomerular tone. ATP is also released locally in the kidney in response to increased perfusion pressure, and in P2X1 receptor knockout mice auto-regulatory responses in kidney afferent arterioles are abolished, indicating an important role in renal glomerular pressure regulation [32, 33].
P2 receptor-mediated regulation of the circulation. See text for details. Purines and pyrimidines are released on the luminal side from endothelial cells, platelets and red blood cells (RBC) in response to hypoxia, acidosis, adrenaline, shear stress and other stimuli. When the endothelial cell layer is intact, the response is vasodilatation by the endothelial release of nitric oxide (NO), endothelium-derived hyperpolarising factor (EDHF) and prostaglandins (Pgl). When the endothelium is damaged, platelets accumulate, release ATP and ADP and mediate vasoconstriction via P2 receptors on the vascular smooth muscle cell (VSMC). On the adventitial side sympathetic and sensory nerves mediate vasoconstriction. Extracellular nucleotides are rapidly degraded by NTPDase1 on endothelium reducing ATP to AMP, followed by conversion to adenosine by CD73 (not shown). In the subendothelium NTPDase2 is present, degrading ATP to ADP, maintaining the platelet activating and contractile effects. Ado adenosine, CGRP calcitonin gene-related peptide, SP substance P
Sympathetic nerves express inhibitory P2Y and P1 (A1) receptors indicating a P2 receptor-mediated negative feedback loop both directly by ATP and its degradation product adenosine [34, 35]. Ectonucleotidases are released from sympathetic nerves together with its substrate ATP, as a termination mechanism for the signalling [36]. Another balancing mechanism is the sympathicolytic effect seen in exercising skeletal muscle, which is important for increased blood flow in the working skeletal muscle during exercise, despite sympathetic NA release that normally would reduce blood flow. This sympathicolytic effect is mimicked by injection in the arterial lumen of ATP and UTP, inducing a vasodilatation that overrides sympathetic vasoconstrictor activity in human skeletal muscle, an effect not obtained by injection of adenosine [37], suggesting that ATP and UTP may mediate this important physiological mechanism.
The first evidence of the presence of P2 receptor subtypes suggested P2X receptors on vascular smooth muscle cells (VSMC) and P2Y receptors on endothelial cells [38]. Rat blood vessel immunohistochemistry and human mRNA quantification have shown the P2X1 receptor to be the highest expressed subtype in smooth muscle cells [39, 40]. It has been difficult to prove the importance of other contractile P2X receptors besides P2X1 due to the paucity of specific agonists and antagonists. However, contractile effects of UTP suggested that P2Y receptors were present on VSMCs and mediated contraction [41]. Using selective pyrimidines resistant to degradation, it has been possible to show that contractile effects are mediated by both UTP-sensitive P2Y2 and UDP-sensitive P2Y6 receptors [42]. Ectonucleotidases in the vessel wall rapidly degrade nucleotides and markedly reduce their contractile effects. Pyrimidine analogues more resistant to ectonucleotidases are powerful vasoconstrictors and up to 1,000-fold more potent than the endogenous ligands [42]. Similarly, a prominent more potent UDP contraction has been seen in NTPDase1 knockout mice, demonstrating the protective effect of ectonucleotidases against nucleotide contraction [43]. In human saphenous vein grafts, used during coronary bypass, a stable UDP analogue stimulates a strong contraction lasting for hours, which is not desensitised [44]. The reason why the P2Y6 receptor is not desensitised is the lack of serine residues in the C-terminal part of the P2Y6 receptor [45]. Neither α1-adrenoceptors, nor P2Y6 receptors, are present in human coronary arteries, possibly to avoid deleterious vasospasm during ischaemia [44].
VSMC express P2Y12 receptors that mediate contraction after stimulation with ADP [46]. At the mRNA level, the P2Y12 receptor is the highest expressed ADP receptor and the second highest expressed P2 receptor in human VSMC [46]. The contractions are not inhibited in patients medicated with clopidogrel. However, drugs with antagonistic effects on P2Y12 receptors that reach the peripheral circulation (AZD6140 and INS50589), affecting both platelets and VSMC, could be of double therapeutic benefit in their prevention of both thrombosis and vasospasm [46].
In conclusion, extracellular nucleotides mediate vasoconstriction when released from nerves on the adventitial side, or when released in the lumen when the endothelium is damaged (Fig. 1). The most important contractile receptors on the VSMC are the ATP P2X1 receptor, the ATP/UTP P2Y2 receptor, the UDP P2Y6 receptor and the ADP P2Y12 receptor (Fig. 1 and Table 2). To determine the relative physiological importance of these receptor subtypes in different human vascular beds is an important task for the future.
Endothelial regulation
Shear stress and hypoxia are important stimuli of both ATP and UTP release from endothelial cells [47] (Fig. 1). Extracellular nucleotides have several important effects mediated by activation of endothelial cells. Vasodilatation and decreased blood pressure by release of prostaglandins and NO has been demonstrated in several studies [5], but P2 receptors also mediate release of endothelium-derived hyperpolarising factor (EDHF), which relaxes VSMC by activation of potassium channels, with subsequent hyperpolarisation [48–50]. Both UTP and ATP reduce forearm vascular resistance in a prostaglandin and NO independent way [51], indicating an important role for EDHF in P2 receptor-mediated vasodilatation in man.
The P2Y1 receptor seems to be of major importance in most vascular beds [11]. However, several other P2 receptors are important for endothelial regulation. Pharmacology of vasodilatation and mRNA quantification in man indicates that P2Y2 and to a lesser degree P2Y6 also are important endothelial P2Y receptors [40, 52]. Knockout mice experiments recently confirmed this picture and demonstrated that the P2Y4 receptor does not mediate dilatation [53]. UTP and ATP are equipotent as vasodilators when infused in the human forearm circulation, indicating a role for both purinergic and pyrimidinergic receptors.
The P2X1 receptor is not expressed on the vascular endothelium and the evidence for other P2X receptors has been scarce, except for the P2X4 receptor which is the highest expressed P2 receptor in endothelium [40, 54]. Using antisense oligonucleotides the P2X4 receptor was shown to be important for shear stress-dependent Ca2+ influx via an ATP-dependent mechanism [55]. This indicates that ATP and P2 receptors may be of importance for shear stress-mediated effects, which is in agreement with the well-established release of ATP from endothelial cells during shear stress [56]. Vessel dilation induced by acute increases in blood flow is markedly suppressed in P2X4 receptor knockout mice [57]. Thus, endothelial P2X4 channels are crucial to flow-sensitive mechanisms that regulate blood pressure and vascular remodelling [57].
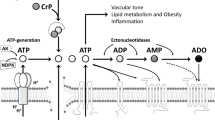
Extracellular ATP in the circulation is rapidly degraded into ADP, AMP and adenosine by ectonucleotidases. Vascular NTPDase1 (CD39) is an endothelial cell membrane protein with both ecto-ATPase and ecto-ADPase activities [8, 58]. Ectonucleotidases are also released by shear stress from endothelial cells [59].
Reactive hyperaemia is the massive increase in blood flow that starts when a blood vessel is opened after a period of ischaemia. It is well known that ATP is released during ischaemia [7, 60]. Adenosine only mediates the late phase of the reactive hyperaemia as has been shown by infusion of adenosine deaminase or a selective A2A antagonist [61, 62]; ADP has been shown to mediate the mid-portion and peak of coronary reactive hyperaemia via endothelial P2Y1 receptors [63]. It has been proposed that different purines may mediate three phases of the reactive hyperaemia [63]. According to this hypothesis, ATP would mediate the first part of the hyperaemia via endothelial P2Y2 or P2X4 receptors. ATP would then be degraded to ADP, which mediates peak hyperaemia via endothelial P2Y1 receptors, followed by degradation of ADP to adenosine resulting in late phase hyperaemia mediated via A2A receptors on smooth muscle cells.
Red blood cells as regulators of vascular tone
The matching of oxygen supply with demand requires a mechanism that increases blood flow in response to decreased tissue oxygen levels. Several reports suggest that the red blood cell (RBC) acts as a sensor for hypoxia and different mechanisms have been suggested by which the deoxygenated RBC stimulates vasodilatation [37, 64–66]. RBCs contain millimolar amounts of ATP and possess the membrane-bound glycolytic enzymes necessary for its production [67–69]. ATP is released in response to reductions in oxygen tension and pH [37, 64] (see Table 3). It has been shown in vitro that vessels dilate in response to low O2 levels only when blood vessels are perfused with RBCs [70]. ATP is released in working human skeletal muscle circulation depending on the number of unoccupied haemoglobin O2 binding sites [64, 71]. Similar results have been shown in the coronary circulation of dogs [72]. The released ATP then binds to P2Y receptors on the endothelium and stimulates vasodilatation. Thus, the RBC functions as an O2 sensor, contributing to the regulation of blood flow and O2 delivery, by releasing ATP depending on the oxygenation state of haemoglobin.
ADP activates a negative feedback pathway for ATP release from human RBCs via P2Y13 receptors [73]. Since blood consists of approximately 40% RBCs that contain a 1,000-fold higher ATP concentration than plasma (mmol/l vs μmol/l), even a minor release of ATP from the high intracellular concentrations could have major circulatory effects. A negative feedback system may therefore be of great physiological importance to mitigate ATP release. Another negative feedback system is the mechanism by which NO inhibits ATP release from erythrocytes, illustrating how NO may turn off ATP release [74]. NO is then scavenged by haemoglobin.
Furthermore, the previous view of the RBC as a “passive bag that transports oxygen” is challenged. It now turns out that it releases ATP in response to stimuli and, as with most important signalling systems, it has a negative feedback system to terminate its release. The described negative feedback pathway may be important to avoid high extracellular concentrations of ATP. At levels above 100 µmol/l, ATP concentrations may exceed the catalytic capacity of ectonucleotidases and could, in fact, stimulate ATP release by increasing permeability of the RBC [75], probably via P2X7 receptors [76]. ATP may even release ATP from endothelial cells [77]. At high concentrations of ATP, a self-sustaining process may thus be instigated which may contribute to the irreversible stage of circulatory shock that can develop rapidly in severely ill patients. Similar mechanisms may be of importance in malaria because induction of the osmolyte permeability in Plasmodium-infected erythrocytes involves purinoceptor signalling [78].
A mechanism of ATP release from RBCs, which has eluded researchers for many years, has recently been suggested by Locovei and co-workers as being mediated via the gap junction protein pannexin-1 [79]. Erythrocytes do not form gap junctions, instead pannexin-1 forms a mechanosensitive ATP-permeable channel that mediates osmotically induced ATP release.
Hypertension
Blood pressure regulation by purinergic signalling is the net result of balancing contractile and dilatory effects as described above. ATP and UTP released on the luminal side, from endothelial cells and erythrocytes, stimulate vasodilatation, in contrast to release from nerves on the adventitial side, which results in vasoconstriction. ATP may also regulate blood pressure via renal mechanisms or brain stem regulation [31, 32].
ATP plays a significantly greater role as a sympathetic cotransmitter in spontaneously hypertensive rats (SHR) [80, 81], and there is increased responsiveness of the renal vasculature of isolated perfused rat kidneys to α,β-methylene ATP in SHR [82], while mesenteric vascular contractile reactivity to ATP via P2X1 and P2Y2 receptors is not altered in deoxycorticosterone acetate (DOCA)-salt hypertension [83]. In the aorta of SHR, endothelium-dependent relaxation to ATP is impaired because of the concomitant generation of an endothelium-derived contracting factor. Contractions to ATP were significantly potentiated in SHR aorta [84]. In hypertensive mature stroke-prone rats, NPY modulation of release of NA and ATP from sympathetic perivascular nerves in kidney vessels is impaired, which may account for the increased nerve-mediated responses [85]. Thus, animal models of hypertension reveal several alterations in purinergic signalling that may contribute to the development of hypertension.
Diadenosine polyphosphates such as Ap4A, Ap5A and Ap6A are combinations of two adenosine molecules connected with four to six phosphate groups. They have been identified as vasocontractile agents [86], probably via actions on P2X1 and P2Y2 receptors. Ap5A and Ap6A are stored at higher levels in platelets from patients with hypertension and may contribute to their increased peripheral vascular resistance [87]. Up4A is a novel endothelium-derived vasoconstrictive factor more potent than endothelin in renal vasoconstriction [88]. It is released upon stimulation of the endothelium by acetylcholine, thrombin and mechanical stress and can be cleaved into either ATP or UTP to stimulate both P2X1 and P2Y2 receptors on VSMC resulting in increased blood pressure [88].
As mentioned above, the P2X4 receptor is the most abundantly expressed P2 receptor in the endothelium and mediates shear stress-stimulated vasodilatation. P2X4 receptor knockout mice have higher blood pressures and excrete smaller amounts of NO products in their urine than do wild-type mice [57]. The P2X4 receptor protein is upregulated in the placenta in preeclampsia [89]. Thus, shear stress-stimulated release of ATP acting on P2X4 receptors may be important for blood pressure regulation.
The importance of P2 receptors for hypertension will not be proven until tested in man. It would be of great interest to perform a clinical study on the effects of P2X1, P2Y2 or P2Y6 receptor antagonists in the treatment of hypertension.
Pulmonary hypertension
Erythrocyte release of ATP appears to regulate pulmonary resistance under some conditions [90], and patients with pulmonary hypertension have been found with impaired release of ATP from RBC [69]. Endothelium-dependent relaxation to ATP has been demonstrated in human pulmonary arteries [91]. On the other hand, ATP is a mitogen for pulmonary artery smooth muscle cells, which may be relevant for the pathophysiological basis of pulmonary hypertension [92]. Again, the balance between endothelial versus smooth muscle stimulation by ATP may regulate blood pressure in different directions and we are far from a full understanding of the role of the purinergic system in pulmonary hypertension.
Migraine and vascular pain
Classic migraine is associated with two distinct vascular changes: an initial vasoconstriction, followed by vasodilatation associated with pain. It has been proposed that purinergic signalling is involved in these changes. ATP released from perivascular sympathetic nerves may participate in producing the initial vasospasm mediated by P2 receptors in vascular smooth muscle. Cerebral arteries are strongly contracted by UTP and especially UDP via P2Y6 receptors. A UDP agonist might therefore have similar effects as the 5-HT1D agonists frequently used to treat migraine [93]. ATP released by endothelial cells acting on P2 receptors on endothelial cells mediating release of NO and producing vasodilatation may contribute to the phase of reactive hyperaemia following vasospasm [94]. It has been suggested that antagonists to P2X3 receptors, which are located on nociceptive sensory nerve endings in cerebral vessels, may be promising candidates for anti-migraine drug development [95]. A recent report demonstrates selective upregulation of nociceptive P2X3 receptors on trigeminal neurons by calcitonin gene-related peptide and suggests that this mechanism might contribute to pain sensitisation in migraine [96]. P2Y receptors on trigeminal sensory nerves have also been implicated in pain in migraine. It has been claimed that migraine attacks are characterised by a relative depletion of sympathetic NA stores in conjunction with an increase in release of ATP [97]. In a review in the Lancet in 1996, Burnstock proposed that vascular pain, as in angina, ischaemic muscle and pelvic pain in women, may be initiated by ATP released from microvascular endothelial cells during reactive hyperaemia, diffusing a short distance to activate P2X3 nociceptive receptors on sensory nerves in the adventitia [26].
Atherosclerosis
Atherosclerosis is the main cause of ischaemic stroke and cardiovascular disease and is now considered to be an inflammatory disease [98]. The formation of a plaque starts with the accumulation of cholesterol followed by invasion of macrophages taking up cholesterol and becoming foam cells. The plaque can be stabilised by smooth muscle cell formation of a fibrous cap that covers the lipid-rich region. However, stimulation of inflammation by oxidised low-density lipoprotein activates macrophages and dendritic cells into antigen-presenting cells, activating T lymphocytes resulting in release of cytokines and metalloproteinases degrading the fibrous cap. The end result is a vulnerable plaque and, when it ruptures, its highly thrombogenic contents activates platelets and causes the formation of a local thrombus occluding the artery or embolising, resulting in ischaemic stroke or myocardial infarction. Evidence both from basic research and from clinical studies indicates important involvement on several levels for purinergic signalling in the atherosclerotic process (Fig. 2). Interestingly, fish oil components increase the release of ATP [99] and a high cholesterol diet decreases ATP release from arteries [100, 101].
Functional roles of P2 receptors in the atherosclerotic inflammatory plaque and during restenosis. See text for details. Purines and pyrimidines acting on P2 receptors stimulate vascular inflammation both by actions on the endothelial cell (EC) and by effects on inflammatory cells. Furthermore, they stimulate vascular smooth muscle cell (VSCM) proliferation, the conversion to synthetic phenotype and production of matrix proteins. Mitogenic P2 receptors are upregulated by growth factors and cytokines. IL interleukin, MCP-1 monocyte chemoattractant protein-1, ICAM-1 intercellular adhesion molecule-1, TSP thrombospondin, IDO indoleamine 2,3-dioxygenase
Atherosclerosis—P2 receptor-mediated effects on inflammatory cells
Inflammatory cells express a large number of P2 receptors with multiple effects [102]. The final result is difficult to predict depending on the subtype of receptor expressed by a particular cell type and on the differentiation stage of the cell. The most important inflammatory cells for atherosclerosis are monocytes that differentiate into macrophages or dendritic cells in the plaque, and the T-helper and suppressor lymphocytes that coordinate the inflammatory reaction in the plaque [98].
A large number of P2 receptors are expressed on T lymphocytes and macrophages [14, 103] and have been suggested to be important players in atherosclerosis [14]. Strong evidence for a functional role exists for P2X7, P2Y2 and P2Y11 receptors. The P2X7 receptor is mitogenic and anti-apoptotic for T lymphocytes [104, 105]. P2X7 is important for release of interleukin (IL)-1 [106], tumour necrosis factor [107] and L-selectin, an adhesion molecule important for lymphocyte binding to endothelium [108]. All of these effects are known to be important for atherosclerosis development. The severity of arthritis is reduced in P2X7 receptor knockout mice [109] and rheumatoid arthritis is coupled to increased incidence of myocardial infarction. It would be of great interest to examine the P2X7 receptor knockout mouse in an atherosclerosis model. ATP and UTP are chemotactic for dendritic cells probably via the P2Y2 receptor and may attract inflammatory cells to the vascular lesion [110]. The P2Y2 receptor enhances the oxidative burst in human macrophages [111]. P2Y1 receptor deletion in knockout mice reduces the atherosclerotic lesions and the plaque area occupied by macrophages in ApoE knockout mice [112]. Whether this is due to platelet inhibition, endothelial or an inflammatory mechanism requires further studies.
ATP inhibits CD4+ T cell activation via an increase in cyclic AMP (cAMP) probably via a P2Y11 receptor [113]. ATP acting on P2Y11 receptors regulates the maturation of human monocyte-derived dendritic cells and induces immunosuppression by inhibiting T-helper 1 cytokines and promoting T-helper 2 cytokines [114, 115].
A polymorphism in the P2Y11 receptor has been shown to have clinical importance by increasing the risk of myocardial infarction [116]. The G-459-A polymorphism, carried by one fifth of the population, causes an Ala-87-Thr substitution in the P2Y11 ATP receptor and increases the risk of myocardial infarction by 21%. The odds ratio increased stepwise depending on the number of Thr-87 alleles, and in subgroups in which the genetic influence is known to be of increased importance, family history of acute myocardial infarction (AMI), early onset AMI or the combined group of early onset AMI with family history. The mechanism by which the polymorphism causes AMI seems to be coupled to increased inflammation because the Thr-87 variant of the P2Y11 receptor was coupled to elevated C-reactive protein (CRP) levels. CRP is a marker of inflammation and an independent prognostic risk factor for the development of AMI [98]. Thus, the P2Y11 receptor is important in the development of atherosclerosis via modulation of inflammation either via effects on T lymphocytes or macrophage cells.
Proinflammatory effects on the endothelium
Even though P2 receptor-mediated activation of the endothelium stimulates release of NO, which inhibits inflammatory cells, it is now well established that important proinflammatory endothelial effects may also be triggered. A proinflammatory dysfunctional endothelium is crucial in the recruitment of monocytes to the atherosclerotic plaque and in the general extravasation and loss of peripheral resistance seen in sepsis. ATP stimulates neutrophil adherence to cultured endothelial cells [117]. ATP stimulates release of IL-6, IL-8, monocyte chemoattractant protein-1, growth-regulated oncogene α and increased expression of intercellular adhesion molecule-1 in human microvascular endothelial cells [118]. UTP and ATP stimulate expression of proinflammatory vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells through activation of the P2Y2 receptor and increases the adherence of monocytic cells to human coronary endothelial cells, an effect that was inhibited by anti-VCAM-1 antibodies [119]. VCAM is important for the recruitment of monocytes and lymphocytes. The VCAM stimulation is caused by P2Y2 receptor-induced transactivation of the vascular endothelial growth factor receptor-2 [120]. These effects have been confirmed in an in vivo neointima model, in which perivascular infusion of UTP enhanced infiltration by macrophages [121].
In conclusion, ATP and UTP stimulate several inflammatory responses known to be important for atherosclerosis development (Fig. 2).
Restenosis and vascular smooth muscle cell proliferative disease
VSMC proliferation contributes to plaque development, but it is probably beneficial by stabilising the plaque and forming a stronger fibrinous cap. However, there are several situations in which VSMC proliferation is important in vascular disease development: restenosis after balloon angioplasty, diabetic microvascular disease, chronic allograft rejection, pulmonary hypertension and possibly systemic hypertension. The latter is suggested since sympathetic nerves exert a trophic effect on vascular smooth muscle [122]. ATP is a cotransmitter from sympathetic nerves [25] and has been shown to be a more potent mitogen than the other cotransmitters NA and NPY [123].
Extracellular ATP is a potent growth factor for VSMC by activation of G protein-coupled P2Y receptors [12, 123–125]. UTP is also mitogenic, acting on P2Y2 receptors [123], as is UDP acting on P2Y6 receptors [124, 126, 127]. ATP is synergistic with polypeptide growth factors (e.g. platelet-derived growth factor, basic fibroblast growth factor) and insulin [123, 124]. The signal transduction is mediated via Gq proteins, phospholipase C and D, diacylglycerol, protein kinase C, extracellular signal-regulated kinase, phosphatidylinositol-3 kinase, MAPK/ERK activity kinase, mitogen-activated protein kinases and Rho [12, 128, 129]. Several immediate early genes are activated and the cell is taken through different phases of the cell cycle [125, 130]. Sometimes, as with UDP acting on P2Y6 receptors, progression is stimulated to both the S and G2 phases, that is through the whole cell cycle [127].
Mitogenic effects have been demonstrated in rat, porcine, and bovine VSMC and cells from human coronary arteries, aorta, and subcutaneous arteries and veins [12, 13, 124, 131]. The trophic effects on VSMC and the abundant sources for extracellular ATP in the vessel wall make a pathophysiological role probable in the development of atherosclerosis, neointima formation after angioplasty, chronic allograft rejection, pulmonary hypertension, diabetic microvascular disease and possibly hypertension. In these processes interaction between the VSMC and the matrix is important. It is therefore interesting that the human P2Y2 receptor contains an integrin-binding domain (RGD) in its first extracellular loop and that interaction with integrins influences P2Y2 receptor-mediated activation of G proteins [132]. ATP stimulates release of matrix metalloproteinase-2 (MMP-2) from human aortic smooth muscle cells and MMP-2 has been implicated in aortic aneurysm pathogenesis [133]. MMPs open up the matrix for migrating VSMC. ATP and UTP are potent chemotactic agents stimulating VSMC migration via P2Y2 and P2Y6 receptors [134]. These mechanisms, together with growth-stimulating effects, explain why perivascular infusion of UTP in a neointima model enhanced neointimal development [121]. Overexpression of the ATP- and UTP-degrading enzyme NTPDase1 reduces neointima development in rat aorta [135]. Augmentation of NTPDase1 activity is an important adaptive response for cardiac allograft survival, with increased inflammation, platelet deposition and infarction rates in NTPDase-deficient mice [136]. The mechanism is dependent on increased expression of the atherosclerotic matrix protein osteopontin [134, 137].
Diabetic microvascular disease
Sympathetic vascular dysfunction in early experimental juvenile diabetes was recognised many years ago. Two weeks after induction of streptozotocin diabetes in rats, there was prejunctional impairment of sympathetic neurotransmission and impaired ATP-mediated endothelial function in mesenteric arteries [138]. Altered relaxant responses to ATP in the corpus cavernosum of men and rats with diabetes have also been reported [139].
As mentioned above, red blood cells may stimulate vasodilatation via release of ATP, which may be important in maintaining perfusion in the microcirculation. Interestingly, in erythrocytes from humans with type 2 diabetes ATP release is impaired, which is consistent with the hypothesis that the defect in erythrocyte physiology could contribute to the vascular disease associated with this clinical condition [140].
High extracellular glucose releases ATP and/or UTP in endothelial cells and pancreatic β cells [141, 142]. An increase in glucose from 5 to 15 mmol/l results in a marked increase in the proatherogenic nuclear factor of activated T cells signalling pathway in VSMC [143]. The effect is mediated via glucose-induced release of ATP and UTP, which subsequently activate P2Y2 but also P2Y6 receptors (after degradation to UDP). Thus, nucleotide release is a potential metabolic sensor for the arterial smooth muscle response to high glucose. Diabetic patients experience microvascular disease characterised by increased wall-lumen ratio, mainly because of an increase in VSMC and have higher rates of restenosis after coronary angioplasty. High glucose-induced release of extracellular nucleotides, acting on P2Y receptors to stimulate VSMC growth via NFAT (nuclear factor of activated T cells) activation, may provide a link between diabetes and diabetic vascular disease [143].
In conclusion, ATP/UTP/UDP stimulate VSMC growth, migration, release of MMPs and osteopontin that may contribute to the development of restenosis, diabetic microvascular disease, chronic allograft rejection, pulmonary hypertension and possibly systemic hypertension (Fig. 2).
Vascular remodelling and angiogenesis
Vascular remodelling
P2 receptors may regulate VSMC phenotype and vice versa, and P2 receptor expression is markedly altered during phenotype changes [13]. The shift from a specialised contractile VSMC phenotype into a proliferating, matrix-producing, synthetic phenotype is a prerequisite for VSMC pathogenesis in vascular disease. Pacaud and co-workers found that the Ca2+-mobilising effects of 2-methylthioATP increased in VSMC during culture in serum, indicating upregulation of P2Y1 receptors in the transition from contractile to synthetic phenotype [144]. This was confirmed at the mRNA level where P2Y2 receptors were found to be upregulated, while P2X1 receptors were downregulated [145]. Mitogenic P2Y receptors are upregulated while ion channel receptors, with only contractile effects, are downregulated in the synthetic phenotype [145]. Growth factors, cytokines and interestingly also ATP are potent stimulators of P2Y2 receptor expression [146, 147]. Thus, factors of importance in the development of vascular disease increase mitogenic P2Y2 receptors; this is further supported by their upregulation in neointima after balloon angioplasty [148, 149]. ATP has a dual concentration-dependent effect on VSMC phenotype. Low ATP concentrations stimulate expression of genes specific for the contractile phenotype, while high ATP concentrations cause a phenotypic shift from the contractile to the synthetic phenotype, and this shift is dependent on a transient activation of protein kinase A, which inhibits activation of a serum response factor [150]. This previously unrecognised mechanism also appears to be important for the profound mitogenic effect of ATP. In intact human blood vessels examined in vitro, shear stress decreased contractile P2X1 receptor expression, but increased the expression of mitogenic P2Y2 and P2Y6 receptors in VSMC [151]. This mechanism could promote vascular growth and remodelling induced by shear stress as an adaptive response to increased flow. P2X4 receptor knockout mice are incapable of adaptive vascular remodelling, that is, a decrease in vessel size in response to a chronic decrease in blood flow [57].
Angiogenesis
Very little is known about the role of P2 receptors in angiogenesis, but there are some studies suggesting their involvement. Ischaemia stimulates release of ATP [7, 60] and it has been shown to be a growth factor for endothelial cells [13, 152]. ATP and UTP stimulate cytoskeletal rearrangements with consequent cell migration of human endothelial cells [153] and ATP stimulates vasa vasorum neovascularisation in pulmonary arteries [154]. Newly developed vascular endothelia express very high levels of NTPDase1, also seen under hypoxic conditions [155]. NTPDase1 knockout mice exhibit disordered cellular migration and angiogenesis [156].
Varicose veins
It has been suggested that cell lysis, consequent to P2X7 receptor-induced pore formation, contributes to the disorganisation and decrease in contractile myocytes in the media of varicose veins [157]. Upregulation of P2Y1 and P2Y2 receptors and downregulation of P2X1 receptors on smooth muscle of varicose veins is associated with a shift from contractile to synthetic and/or proliferative roles; this phenotypic change in smooth muscle leads to weakening of vein walls and may be a causal factor in the development of varicose veins [158], an interesting parallel to the receptor changes seen in response to increased shear stress (see above) [151].
Heart
ATP is released in the heart as a cotransmitter together with catecholamines from sympathetic nerves, but it may also be released from other sources in the heart such as endothelium, platelets, RBC and ischaemic myocardium (Table 3) [7, 60, 159]. P2 receptors are abundantly expressed in the foetal human heart [160] as well as in the adult human heart [60, 161].
Inotropy
In cardiomyocytes, ATP stimulates a pronounced positive inotropic effect and may also act in synergy with β-adrenergic agonists to augment myocyte contractility [60, 162–165]. ATP stimulates an increase in cytosolic calcium and evidence for the involvement of inositol 1,4,5-trisphosphate (IP3)-coupled P2Y2 receptors and ion channel P2X receptors has been presented [162, 164–167]. Ap4A, probably after degradation to ATP, increases force of contraction in human ventricular trabeculae [168].
The inotropic effects of ATP are dependent on both IP3 and cAMP [169]. The inotropic effects of catecholamines acting on β-receptors are mediated by an increase of cAMP and antagonists of these receptors are important drugs for the treatment of hypertension and reduce mortality in congestive heart failure. Similarly, ATP stimulates an increase in cAMP in cardiac myocytes and may act in synergy with the β1-adrenergic agonist, isoproterenol, by differential activation of adenylyl cyclase isoforms [60, 170, 171]. However, the ATP receptor mediating this increase in cAMP has not been related to any particular P2 receptor subtype in cardiac cells [60]. It could be the P2Y11 receptor that is additionally coupled to Gs and activates adenylyl cyclase [60, 172–174]. mRNA for the P2Y11 receptor has been detected in the human heart at high levels [161, 175].
The unstable agonist, UTP, has been shown to induce a positive inotropic effect in rat atria and in rat and guinea pig ventricular cardiomyocytes [164, 166, 176, 177]. Stable UTP and UDP analogues induce a pronounced inotropic effect on mouse cardiomyocytes [161]. The P2Y2 receptor is the most abundantly expressed receptor with very low levels of the P2Y4 receptor in the human heart [161], suggesting that the inotropic effects of UTP are mediated via P2Y2 receptors, while the UDP effects are mediated via the P2Y6 receptor [161]. These mechanisms are mediated via IP3-mediated signalling.
In conclusion, the inotropic effects in man are mediated via P2Y2 and P2Y6 receptors and a P2Y11-like receptor (Fig. 3). P2X receptors are probably also involved, since several subtypes are expressed [60, 160], but it has not been possible to perform a clear receptor characterisation due to lack of selective antagonists.
Functional roles of purines and pyrimidines acting on P2 receptors in the regulation of the heart. See text for details. a ATP, UTP and UDP exert inotropic effects on cardiomyocytes leading to increased cardiac output. b UTP and UDP stimulate hypertrophy of cardiomyocytes, while ATP can have apoptotic effects. c UTP protects against ischaemic injury and cardiomyocyte cell death. ATP-degrading enzymes preserve endothelial integrity and protect against allograft rejection. NTPDase nucleoside triphosphate diphosphohydrolase
Myocardial infarction
Using microdialysis, ATP in the interstitial space has been estimated to be 40 nmol/l, but the levels may increase markedly during electrical stimulation, ischaemia, challenge with cardiotonic agents, increase in blood flow, mechanical stretch and increased work load [60]. ATP is released from cardiomyocytes during reduced oxygen tension [60]. UTP and ATP are released from the heart during cardiac ischaemia [178] and patients with myocardial infarction have higher plasma levels of both ATP and UTP [161, 179]. There is a significant increase in ectonucleotidase activity (NTPDase1) in the hearts of patients with ischaemic heart disease [180] that could represent a compensatory mechanism against increased nucleotide levels during chronic ischaemia.
Ischaemia-reperfusion provokes barrier failure of the coronary microvasculature, leading to myocardial oedema, ATP may protect against reperfusion-induced coronary endothelial barrier damage and inhibition of ATP degradation enhances the stabilising effect of ATP on barrier function [181].
The common P2Y11 ATP receptor polymorphism, Ala-87-Thr, is associated with both increased CRP and increased risk of developing myocardial infarction [116]. Based on this association, we hypothesise that the P2Y11 receptor plays an important role in cardiovascular biology and in inflammatory disease (see above), causing myocardial infarction via a proinflammatory effect. However, increased inotropic effects may also contribute.
An interesting yin and yang situation for ATP and UTP has been revealed regarding hypertrophic effects. UTP but not ATP causes hypertrophic growth in neonatal cardiomyocytes [182] (Fig. 3). In contrast, ATP inhibits hypertrophy and may even induce apoptosis and necrosis [163, 183]. The reason for the difference in effects could be due to activation of P2X receptors or cAMP stimulation by ATP [60, 161]. Both UTP and ATP transactivate epidermal growth factor receptors, but only ATP stimulates the hypertrophic marker genes atrial natriuretic peptide and myosin light chain 2 [184]. Similarly, UTP but not ATP protects cultured cardiomyocytes against hypoxic stress [185]. Since UTP is released during preconditioning [178], a role for UTP in the protective effects of preconditioning is plausible. Recently, Yitzhaki and co-workers were able to demonstrate prominent reductions in myocardial infarction size and improved rat heart function in vivo, by a single intravenous bolus dose of UTP before ischaemia [186] (Fig. 3).
Congestive heart failure
There are several reports of alterations or adaptations of purinergic signalling during congestive heart failure. The positive inotropic effect of ATP is impaired in heart failure, but reversed by the angiotensin-converting enzyme (ACE)inhibitor imidapril [187]. The contractile responses for the P2Y11 receptor agonist AR-C67085 are decreased in heart failure, suggesting a downregulation of this receptor function in cardiomyocytes in a similar manner as seen for β1-receptors in congestive heart failure [169]. In human hearts, only the P2X6 receptor was altered in congestive heart failure [188]. In congestive heart failure P2X1 receptors are downregulated in VSMC in resistance arteries, which could represent a protective response against the increased sympathetic nerve activity and peripheral resistance seen in congestive heart failure [189].
Several P2 receptors have been suggested as targets for pharmacological treatment of congestive heart failure. Overexpression of P2X4 receptors has a beneficial, life-prolonging effect in a heart failure model [190]. Both ATP and catecholamines are released from sympathetic nerves, acting through cAMP-stimulating receptors to mediate positive inotropic effects, stimulating the same intracellular mechanisms as adrenergic β-receptors. It is possible that agonists for the P2Y11 receptor could be used to improve cardiac output in patients with circulatory shock. However, an even more important drug candidate would be a P2Y11 receptor antagonist that may be beneficial in patients with congestive heart failure. The extracellular pyrimidines UTP and UDP may be inotropic factors in man, acting on P2Y2 and P2Y6 receptors stimulating the same intracellular pathways as angiotensin II. Synthetic agonists could thus be used as inotropic agents during circulatory shock and antagonists may have effects similar to angiotensin II receptor blockers, being beneficial in the treatment of hypertension and congestive heart failure.
Chronotropy and arrhythmia
It has been difficult to determine the chronotropic effects of ATP due to the dominating inhibitory effects of adenosine on the AV node. We know that ATP increases the contractile rate in neuron-myocyte co-cultures but the effect is markedly reduced in non-innervated myocyte cultures [191]. ATP could induce arrhythmia based on its increased automaticity and early after-depolarisations [60]. The P2X1 receptor is colocalised with connexin 43 in gap junctions that transmit the contractile stimulus between cardiomyocytes [192]. However, so far no firm evidence exists of an important role for P2 receptors for chronotropy or arrhythmia.
Platelets and coagulation
Platelets
ADP, released from erythrocytes or produced after ectonucleotidase breakdown of ATP released from erythrocytes and endothelial cells, was found to cause platelet aggregation as early as 1961, before the concept of P2 receptors was conceived [193]. Later, the effects were attributed to a single receptor, designated P2T, but it was not until 1998 that the three receptors involved were characterised [194–196]. Two ADP receptors (P2Y12 and P2Y1) and one ion channel ATP receptor (P2X1) are expressed on platelets [197, 198] (Fig. 4). The P2Y12 receptor is coupled to inhibition of cAMP. The molecular identifications of the P2Y12 receptor and generation of knockout mice revealed highly prolonged bleeding times, and their platelets aggregate poorly in response to ADP and display a reduced sensitivity to thrombin and collagen [199]. The P2Y1 receptor is responsible for ADP-induced shape change and weak, transient aggregation [200–202], while the P2Y12 receptor is responsible for the completion and amplification of the response to ADP and to all platelet agonists, including thromboxane A2, thrombin and collagen. The platelets also express P2Y1 receptors that have been shown in knockout mice to be of similar importance as P2Y12 receptors [200, 203].
P2 receptor-mediated regulation of coagulation and platelet aggregation. See text for details. ADP is an important mediator and positive feedback mechanism for platelet aggregation. Activated platelets stimulate coagulation, i.e. formation of a fibrin network. In contrast, ATP, ADP and UTP release tissue plasminogen activator (t-PA) from endothelial cells, resulting in degradation of the fibrin network. At the same time plasminogen activator inhibitor (PAI-1) is also released from endothelial cells by ATP stimulation, in turn inhibiting t-PA. Thus, P2 receptors regulate several balancing factors in haemostasis. Ado adenosine
The physiological importance of the P2X1 receptor is not fully elucidated. However, recent studies have demonstrated that P2X1 receptors can generate significant functional platelet responses, independently and in synergy with other receptor pathways. In addition, studies in transgenic animals indicate an important role for P2X1 receptors in platelet activation, particularly under conditions of shear stress and thus during arterial thrombosis [204]. Synergistic interaction between ATP and NA in stimulating platelet aggregation may have significant clinical implications and suggests a prothrombotic role for ATP in stress [205].
NTPDase1 is an important inhibitor of platelet activation. Unexpectedly, NTPDase1-deficient mice had prolonged bleeding times with minimally perturbed coagulation parameters. Platelet interactions with injured mesenteric vasculature were considerably reduced in vivo and purified mutant platelets failed to aggregate to standard agonists in vitro [206]. This platelet hypofunction was reversible and associated with P2Y1 receptor desensitisation. In keeping with deficient vascular protective mechanisms, fibrin deposition was found at multiple organ sites in NTPDase1-deficient mice and in transplanted cardiac grafts. Endothelial ADPase activity is lost following ischaemia-reperfusion injury, xenograft rejection and inflammation resulting in increased levels of ATP and ADP [58, 207]. This may cause platelet aggregation but also other effects, such as mitogenic effects on VSMC. Infusion of systemic apyrase inhibits platelet aggregation and prolongs xenograft survival [208]. This has also been shown with adenoviral transfer of NTPDase1 [209]. NTPDase1 is also lost in vascular cardiac grafts subjected to oxidant stress. NTPDase2 only degrades ATP to ADP and has a prothrombotic effect. It is present in the subendothelium and can shift the balance in a prothrombotic direction after endothelial injury [210] (Fig. 1).
ADP acting on P2Y12 receptors is not only important for platelet activation, it also stimulates vasoconstriction. Stable drugs with antagonistic effects on P2Y12 receptors, affecting both platelets and VSMC, could be of double therapeutic benefit in their prevention of both thrombosis and vasospasm [46].
Coagulation
The prothrombotic effect of ADP on the platelets is counteracted by the effects of UTP and ATP on the endothelium that stimulates a substantial release of tissue-type plasminogen activator (tPA) with fibrinolytic effects [51, 211] (Fig. 4). To make the picture even more complex, ATP also releases plasminogen activator inhibitor (PAI-1) which in turn inhibits tPA [212]. Thus, P2 receptors are involved at several stages of haemostasis, which is important for the development of atherosclerosis and thrombotic occlusions leading to myocardial infarction and stroke.
Clinical importance of P2 receptor-mediated regulation of platelets
The first ADP inhibitors, ticlopidine and clopidogrel, were developed before the cloning and identification of the platelet ADP receptors. Later, it was concluded that they were prodrugs, converted in the liver to a metabolite that binds irreversibly to the P2Y12 receptor resulting in non-competitive antagonism.
Ticlodipine has the disadvantage of a small risk of neutropaenia, so that clinically so far the most important contribution of drug development aimed at P2 receptors has been the beneficial effects of the platelet ADP receptor antagonist clopidogrel in atherosclerotic disease. In the CAPRIE trial, clopidogrel was even more effective compared to aspirin in preventing myocardial infarctions [213]. Clopidogrel, as an addition to aspirin, turned out to be necessary to prevent acute in-stent thrombosis in percutaneous coronary interventions. The CURE trial demonstrated that the addition of clopidogrel to aspirin reduced clinical events by 20% in acute coronary syndromes [213, 214]. This has also been shown for ST-elevation myocardial infarctions (COMMIT, CLARITY). However, long-term secondary prevention in high-risk groups with clopidogrel added to aspirin did not have significant benefits (CHARISMA). Clopidogrel combined with aspirin reduces cerebral emboli in patients undergoing carotid endarterectomy [215]. Clopidogrel (Plavix®) is now a blockbuster drug and one of the most sold drugs worldwide to the benefit of atherosclerotic patients. Nevertheless, clopidogrel is a rather weak antagonist at P2Y12 receptors with variable effects, often referred to as clopidogrel resistance. Newer P2Y12 receptor antagonists, such as prasugrel and AZD6140, have a stronger more consistent effect and are currently being studied in major clinical trials (TRITON and PLATO).
Clinical importance of purinergic signalling in cardiovascular disease: future directions
The P2 receptor family provides opportunities for drug development (see Table 4). The large number of receptor subtypes and the increasing knowledge of their tissue distributions may make it possible to target a specific cardiovascular organ with limited side effects. So far, only P2Y12 receptor antagonists have been explored in patients, but with extraordinary success. Clopidogrel is one of the most prescribed drugs in the world for the treatment of thrombosis, stroke and myocardial infarctions in millions of patients. The following compounds, prasugrel and AZD6140, are even more potent P2Y12 receptor antagonists with clinical benefits currently being tested in phase III trials. Improved platelet inhibition can also be achieved with antagonists against the other two P2 receptors on platelets. Studies in P2Y1 and P2X1 receptor knockout mice and experimental thrombosis models using selective P2Y1 and P2X1 receptor antagonists have shown that, depending on the conditions, these receptors could also be potential targets for new antithrombotic drugs.
ATP, UTP and UDP, acting on P2Y2 and P2Y6 receptors, are potent stimulators of inflammation, VSMC proliferation and migration. Antagonists to these receptors may be explored to protect against atherosclerosis, restenosis after balloon angioplasty, chronic transplant rejection and diabetic microvascular disease.
It is possible that hypertension and pulmonary hypertension could be treated with P2X1, P2Y2, P2Y6 or P2Y12 receptor antagonists. In the heart P2Y2, P2Y6 and P2Y11 receptor antagonists may have similar beneficial roles as angiotensin inhibition or β-blockers in the treatment of congestive heart failure. In the acute phase of a myocardial infarction, a UTP analogue could protect cardiomyocytes and limit infarct size. A polymorphism in P2Y11 receptors could be considered as a prognostic marker to identify patients at risk of myocardial infarction and drugs aimed at the receptor could be explored to prevent myocardial infarction.
A promising strategy would be to use soluble NTPDase to prevent thrombosis, restenosis and xenograft transplantation. SolNTPDase1 protects against brain damage in a stroke model and a human genetically engineered solNTPDase has been developed that could be used in clinical trials. Possible clinical indications could be stroke, myocardial infarction and transplantation. P2X3 receptor antagonists could be used against migraine and vascular pain [see 19, 26].
References
Drury A, Szent-Györgyi A (1929) The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol 68:213–237
Burnstock G (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW (ed) Cell membrane receptors for drugs and hormones: a multidisciplinary approach. Raven, New York, pp 107–118
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Gordon JL (1986) Extracellular ATP: effects, sources and fate. Biochem J 233:309–319
Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis Found Symp 276:113–128
Burnstock G, Kennedy C (1986) A dual function for adenosine 5’-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res 58:319–330
Olsson RA, Pearson JD (1990) Cardiovascular purinoceptors. Physiol Rev 70:761–845
Ralevic V, Burnstock G (1991) Roles of P2-purinoceptors in the cardiovascular system. Circulation 84:1–14
Erlinge D (1998) Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol 31:1–8
Burnstock G (2002) Purinergic signalling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22:364–373
Di Virgilio F, Solini A (2002) P2 receptors: new potential players in atherosclerosis. Br J Pharmacol 135:831–842
Gachet C (2006) Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol 46:277–300
Agteresch HJ, Dagnelie PC, van den Berg JW et al (1999) Adenosine triphosphate: established and potential clinical applications. Drugs 58:211–232
Ralevic V, Burnstock G (2003) Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect 16:133–140
Atkinson B, Dwyer K, Enjyoji K et al (2006) Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36:217–222
Burnstock G (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58:58–86
Burnstock G (1976) Do some nerve cells release more than one transmitter? Neuroscience 1:239–248
Burnstock G (1988) Sympathetic purinergic transmission in small blood vessels. Trends Pharmacol Sci 9:116–117
Burnstock G (1987) Local control of blood pressure by purines. Blood Vessels 24:156–160
Burnstock G (1988) Local purinergic regulation of blood pressure (The First John T. Shepherd Lecture). In: Vanhoutte PM (ed) Vasodilatation: vascular smooth muscle, peptides, autonomic nerves, and endothelium. Raven, New York, pp 1–14
Burnstock G (1990) Local mechanisms of blood flow control by perivascular nerves and endothelium. J Hypertens Suppl 8:S95–S106
Burnstock G (1990) Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int 17:357–368
Burnstock G (1996) A unifying purinergic hypothesis for the initiation of pain. Lancet 347:1604–1605
Burnstock G (1993) Introduction: changing face of autonomic and sensory nerves in the circulation. In: Edvinsson L, Uddman R (eds) Vascular innervation and receptor mechanisms: new perspectives. Academic, New York, pp 1–22
Rubino A, Burnstock G (1996) Capsaicin-sensitive sensory-motor neurotransmission in the peripheral control of cardiovascular function. Cardiovasc Res 31:467–479
Vial C, Evans RJ (2002) P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 62:1438–1445
Ramme D, Regenold JT, Starke K et al (1987) Identification of the neuroeffector transmitter in jejunal branches of the rabbit mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol 336:267–273
Chan CM, Unwin RJ, Bardini M et al (1998) Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol 274:F799–F804
Inscho EW, Cook AK, Imig JD et al (2004) Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181:445–453
Inscho EW, Cook AK, Imig JD et al (2003) Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112:1895–1905
Boehm S, Huck S (1997) Receptors controlling transmitter release from sympathetic neurons in vitro. Prog Neurobiol 51:225–242
Cunha R (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38:107–125
Todorov LD, Mihaylova-Todorova S, Westfall TD et al (1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387:76–79
Rosenmeier JB, Hansen J, Gonzalez-Alonso J (2004) Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558:351–365
Burnstock G, Kennedy C (1985) Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol 16:433–440
Lewis CJ, Evans RJ (2000) Comparison of P2X receptors in rat mesenteric, basilar and septal (coronary) arteries. J Auton Nerv Syst 81:69–74
Wang L, Karlsson L, Moses S et al (2002) P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 40:841–853
von Kügelgen I, Haussinger D, Starke K (1987) Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol 336:556–560
Malmsjö M, Adner M, Harden TK et al (2000) The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol 131:51–56
Sévigny J et al (2006) Co-ordinated regulation of P2-receptor signaling by membrane bound NTPDases. Purinergic Signalling 2:53
Borna C, Wang L, Gudbjartsson T et al (2003) Contractions in human coronary bypass vessels stimulated by extracellular nucleotides. Ann Thorac Surg 76:50–57
Brinson AE, Harden TK (2001) Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem 276:11939–11948
Wihlborg AK, Wang L, Braun OO et al (2004) ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24:1810–1815
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–342
Malmsjö M, Edvinsson L, Erlinge D (1998) P2U-receptor mediated endothelium-dependent but nitric oxide-independent vascular relaxation. Br J Pharmacol 123:719–729
Malmsjö M, Erlinge D, Högestätt ED et al (1999) Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur J Pharmacol 364:169–173
Mistry H, Gitlin JM, Mitchell JA et al (2003) Endothelium-dependent relaxation and endothelial hyperpolarization by P2Y receptor agonists in rat-isolated mesenteric artery. Br J Pharmacol 139:661–671
Hrafnkelsdottir T, Erlinge D, Jern S (2001) Extracellular nucleotides ATP and UTP induce a marked acute release of tissue-type plasminogen activator in vivo in man. Thromb Haemost 85:875–881
Wihlborg AK, Malmsjö M, Eyjolfsson A et al (2003) Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol 138:1451–1458
Guns PJ, Korda A, Crauwels HM et al (2005) Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol 146:288–295
Yamamoto K, Korenaga R, Kamiya A et al (2000) P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol 279:H285–H292
Yamamoto K, Korenaga R, Kamiya A et al (2000) Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87:385–391
Bodin P, Bailey D, Burnstock G (1991) Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol 103:1203–1205
Yamamoto K, Sokabe T, Matsumoto T et al (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–137
Kaczmarek E, Koziak K, Sévigny J et al (1996) Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem 271:33116–33122
Yegutkin G, Bodin P, Burnstock G (2000) Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5’-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol 129:921–926
Vassort G (2001) Adenosine 5’-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81:767–806
Saito D, Steinhart CR, Nixon DC et al (1981) Intracoronary adenosine deaminase reduces canine myocardial reactive hyperemia. Circ Res 49:1262–1267
Zatta AJ, Headrick JP (2005) Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol 144:576–587
Olivecrona GK et al (2006) Coronary artery reperfusion: the ADP receptor P2Y1 mediates early reactive hyperemia in vivo in pigs. Purinergic Signalling 1:59–64
Ellsworth ML, Forrester T, Ellis CG et al (1995) The erythrocyte as a regulator of vascular tone. Am J Physiol 269:H2155–H2161
McMahon TJ, Moon RE, Luschinger BP et al (2002) Nitric oxide in the human respiratory cycle. Nat Med 8:711–717
Cosby K, Partovi KS, Crawford JH et al (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9:1498–1505
Bergfeld GR, Forrester T (1992) Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26:40–47
Sprague RS (2001) Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281:C1158–C1164
Sprague RS, Stephenson AH, Ellsworth ML et al (2001) Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 226:434–439
Dietrich HH, Ellsworth ML, Sprague RS et al (2000) Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278:H1294–H1298
Gonzalez-Alonso J, Olsen DB, Saltin B (2002) Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91:1046–1055
Farias M, Gorman MW, Savage MV et al (2005) Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 288:H1586–H1590
Wang L, Olivecrona G, Götberg M et al (2005) ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96:189–196
Olearczyk JJ, Ellsworth ML, Stephenson AH et al (2004) Nitric oxide inhibits ATP release from erythrocytes. J Pharmacol Exp Ther 309:1079–1084
Trams EJ, Kauffman H, Burnstock G (1980) A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol 87:609–621
Sluyter R, Shemon AN, Barden JA et al (2004) Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem 279:44749–44755
Bodin P, Burnstock G (1996) ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol 27:872–875
Tanneur V, Duranton C, Brand VB (2006) Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J 20:133–135
Locovei S, Bao L, Dahl G (2006) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103:7655–7659
Vidal M, Hicks PE, Langer SZ (1986) Differential effects of α-β-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol 332:384–390
Brock JA, Van Helden DF (1995) Enhanced excitatory junction potentials in mesenteric arteries from spontaneously hypertensive rats. Pflugers Arch 430:901–908
Fernandez O, Wangensteen R, Osuna A et al (2000) Renal vascular reactivity to P2-purinoceptor activation in spontaneously hypertensive rats. Pharmacology 60:47–50
Galligan JJ, Hess MC, Miller SB et al (2001) Differential localization of P2 receptor subtypes in mesenteric arteries and veins of normotensive and hypertensive rats. J Pharmacol Exp Ther 296:478–485
Yang D, Gluais P, Zhang JN et al (2004) Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol 18:321–326
Vonend O, Turner CM, Chan CM et al (2004) Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66:157–166
Schluter H, Offers E, Brüggemann G et al (1994) Diadenosine phosphates and the physiological control of blood pressure. Nature 367:186–188
Hollah P, Hausberg M, Kosch M et al (2001) A novel assay for determination of diadenosine polyphosphates in human platelets: studies in normotensive subjects and in patients with essential hypertension. J Hypertens 19:237–245
Jankowski V, Tölle M, Vanholder R et al (2005) Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med 11:223–227
Roberts VH, Webster RP, Brockman DE et al (2006) Post-translational modifications of the P2X4 purinergic receptor subtype in the human placenta are altered in preeclampsia. Placenta 28:270–277
Sprague RS, Olearczyk JJ, Spence DM et al (2003) Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 285:H693–H700
Greenberg B, Rhoden K, Barnes PJ (1987) Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol 252:H434–H438
Zhang S, Remillard CV, Fantozzi I et al (2004) ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287:C1192–C1201
Malmsjö M, Hou M, Pendergast W et al (2003) Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol 3:4
Burnstock G (1989) The role of adenosine triphosphate in migraine. Biomed Pharmacother 43:727–736
Waeber C, Moskowitz MA (2003) Therapeutic implications of central and peripheral neurologic mechanisms in migraine. Neurology 61:S9–S20
Fabbretti E, D’Arco M, Fabbro A et al (2006) Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26:6163–6171
Peroutka SJ (2004) Migraine: a chronic sympathetic nervous system disorder. Headache 44:53–64
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Hashimoto M, Shinozuka K, Shahdat HM et al (1998) Antihypertensive effect of all-cis-5,8,11,14,17-icosapentaenoate of aged rats is associated with an increase in the release of ATP from the caudal artery. J Vasc Res 35:55–62
Hashimoto M, Shinozuka K, Tanabe Y et al (1998) Long-term supplementation with a high cholesterol diet decreases the release of ATP from the caudal artery in aged rats. Life Sci 63:1879–1885
Karoon P, Burnstock G (1998) Reduced sympathetic noradrenergic neurotransmission in the tail artery of Donryu rats fed with high cholesterol-supplemented diet. Br J Pharmacol 123:1016–1021
Di Virgilio F, Chiozzi P, Ferrari D et al (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97:587–600
Wang L, Jacobsen SE, Bengtsson A et al (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5:16
Baricordi OR, Ferrari D, Melchiorri L et al (1996) An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 87:682–690
Kawamura H, Aswad F, Minagawa M et al (2005) P2X7 receptor-dependent and -independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol 174:1971–1979
Ferrari D, Chiozzi P, Falzoni S et al (1997) Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159:1451–1458
Suzuki T, Hide I, Ido K et al (2004) Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 24:1–7
Gu B, Bendall LJ, Wiley JS (1998) Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood 92:946–951
Labasi JM, Petrushova N, Donovan C et al (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168:6436–6445
Idzko M, Dichmann S, Ferrari D et al (2002) Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood 100:925–932
Schmid-Antomarchi H, Schmid-Alliana A, Romey G et al (1997) Extracellular ATP and UTP control the generation of reactive oxygen intermediates in human macrophages through the opening of a charybdotoxin-sensitive Ca2+-dependent K+ channel. J Immunol 159:6209–6215
Hechler B et al (2006) Reduced atherosclerotic lesions in P2Y1/ApoE double knockout mice. Purinergic Signalling 2:299
Duhant X, Schandené L, Bruyns C et al (2002) Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J Immunol 169:15–21
Kaufmann A, Musset B, Limberg SH et al (2005) “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem 280:32459–32467
Marteau F, Gonzalez NS, Communi D et al (2005) Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood 106:3860–3866
Amisten S, Melander O, Wihlborg AK et al (2006) Increased risk of acute myocardial infarction and elevated levels of C-reactive protein in carriers of the Thr-87 variant of the ATP receptor P2Y11. Eur Heart J 28:13–18
Dawicki DD, McGowan-Jordan J, Bullard S et al (1995) Extracellular nucleotides stimulate leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol 268:L666–L673
Seiffert K, Ding W, Wagner JA et al (2006) ATPγS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol 126:1017–1027
Seye CI, Yu N, Jain R et al (2003) The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem 278:24960–24965
Seye CI, Yu N, González FA et al (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279:35679–35686
Seye CI, Kong Q, Erb L et al (2002) Functional P2Y2 nucleotide receptors mediate uridine 5’-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation 106:2720–2726
Hart MN, Heistad DD, Brody MJ (1980) Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension 2:419–423
Erlinge D, Yoo H, Edvinsson L et al (1993) Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am J Physiol 265:H1089–H1097
Wang DJ, Huang NN, Heppel LA (1992) Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol 153:221–233
Malam-Souley R, Seye C, Gadeau AP et al (1996) Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J Cell Physiol 166:57–65
Erlinge D, You J, Wahlestedt C et al (1995) Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol 289:135–149
Hou M, Harden TK, Kuhn CM et al (2002) UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol 282:H784–H792
Wilden PA, Agazie YM, Kaufman R et al (1998) ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol 275:H1209–H1215
Sauzeau V, Le Jeune H, Cario-Toumaniantz C et al (2000) P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol 278:H1751–H1761
Miyagi Y, Kobayashi S, Ahmed A et al (1996) P2U purinergic activation leads to the cell cycle progression from the G1 to the S and M phases but not from the G0 to G1 phase in vascular smooth muscle cells in primary culture. Biochem Biophys Res Commun 222:652–658
Erlinge D, Brunkwall J, Edvinsson L (1994) Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept 50:259–265
Erb L, Liu J, Ockerhausen J et al (2001) An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol 153:491–501
Robinson WP 3rd, Douillet CD, Milano PM et al (2006) ATP stimulates MMP-2 release from human aortic smooth muscle cells via JNK signaling pathway. Am J Physiol Heart Circ Physiol 290:H1988–H1996
Chaulet H, Desgranges C, Renault MA et al (2001) Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res 89:772–778
Koziak K et al (2006) Adenovirus-mediated over-expression of CD39 inhibits neointima formation in rat aorta. Purinergic Signalling 2:Addendum
Imai M, Takigami K, Guckelberger O et al (1999) Modulation of nucleoside triphosphate diphosphohydrolase-1 (NTPDase-1)cd39 in xenograft rejection. Mol Med 5:743–752
Pillois X, Chaulet H, Belloc I et al (2002) Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res 90:678–681
Ralevic V, Belai A, Burnstock G (1995) Effects of streptozotocin-diabetes on sympathetic nerve, endothelial and smooth muscle function in the rat mesenteric arterial bed. Eur J Pharmacol 286:193–199
Gür S, Ozturk B (2000) Altered relaxant responses to adenosine and adenosine 5’-triphosphate in the corpus cavernosum from men and rats with diabetes. Pharmacology 60:105–112
Sprague RS, Stephenson AH, Bowles EA et al (2006) Reduced expression of Gi in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55:3588–3593
Parodi J, Flores C, Aguayo C et al (2002) Inhibition of nitrobenzylthioinosine-sensitive adenosine transport by elevated D-glucose involves activation of P2Y2 purinoceptors in human umbilical vein endothelial cells. Circ Res 90:570–577
Hellman B, Dansk H, Grapengiesser E (2004) Pancreatic β-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab 286:E759–E765
Nilsson J, Nilsson LM, Chen YW et al (2006) High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler Thromb Vasc Biol 26:794–800
Pacaud P, Malam-Souley R, Loirand G et al (1995) ATP raises [Ca2+]i via different P2-receptor subtypes in freshly isolated and cultured aortic myocytes. Am J Physiol 269:H30–H36
Erlinge D, Hou M, Webb TE et al (1998) Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun 248:864–870
Hou M, Möller S, Edvinsson L et al (1999) MAPKK-dependent growth factor-induced upregulation of P2Y2 receptors in vascular smooth muscle cells. Biochem Biophys Res Commun 258:648–652
Hou M, Möller S, Edvinsson L et al (2000) Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol 20:2064–2069
Seye CI, Gadeau AP, Daret D et al (1997) Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol 17:3602–3610
Shen J, Seye CI, Wang M et al (2004) Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol 66:1265–1274
Hogarth DK, Sandbo N, Taurin S et al (2004) Dual role of PKA in phenotypic modulation of vascular smooth muscle cells by extracellular ATP. Am J Physiol Cell Physiol 287:C449–C456
Wang L, Andersson M, Karlsson L et al (2003) Increased mitogenic and decreased contractile P2 receptors in smooth muscle cells by shear stress in human vessels with intact endothelium. Arterioscler Thromb Vasc Biol 23:1370–1376
Van Daele P, Van Coevorden A, Roger PP et al (1992) Effects of adenine nucleotides on the proliferation of aortic endothelial cells. Circ Res 70:82–90
Kaczmarek E, Erb L, Koziak K et al (2005) Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost 93:735–742
Gerasimovskaya EV, Davie NJ, Ahmad S et al (2005) Extracellular adenosine triphosphate: a potential regulator of vasa vasorum neovascularization in hypoxia-induced pulmonary vascular remodeling. Chest 128(6 Suppl):608S–610S
Eltzschig HK, Ibla JC, Furuta GT (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198:783–796
Goepfert C, Sundberg C, Sévigny J et al (2001) Disordered cellular migration and angiogenesis in cd39-null mice. Circulation 104:3109–3115
Cario-Toumaniantz C, Loirand G, Ladoux A et al (1998) P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res 83:196–203
Metcalfe MJ, Baker DM, Burnstock G (2006) Purinoceptor expression on keratinocytes reflects their function in the epidermis during chronic venous insufficiency. Arch Dermatol Res 298:301–307
Clemens MG, Forrester T (1981) Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol 312:143–158
Bogdanov Y, Rubino A, Burnstock G (1998) Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci 62:697–703
Wihlborg AK, Balogh J, Wang L et al (2006) Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ Res 98:970–976
Danziger RS, Raffaeli S, Moreno-Sanchez R et al (1988) Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium 9:193–199
Zheng JS, Boluyt MO, Long X et al (1996) Extracellular ATP inhibits adrenergic agonist-induced hypertrophy of neonatal cardiac myocytes. Circ Res 78:525–535
Podrasky E, Xu D, Liang BT (1997) A novel phospholipase C- and cAMP-independent positive inotropic mechanism via a P2 purinoceptor. Am J Physiol 273:H2380–H2387
Mei Q, Liang BT (2001) P2 purinergic receptor activation enhances cardiac contractility in isolated rat and mouse hearts. Am J Physiol Heart Circ Physiol 281:H334–H341
Froldi G, Pandolfo L, Chinellato A et al (1994) Dual effect of ATP and UTP on rat atria: which types of receptors are involved? Naunyn Schmiedebergs Arch Pharmacol 349:381–386
Scamps F, Vassort G (1994) Pharmacological profile of the ATP-mediated increase in L-type calcium current amplitude and activation of a non-specific cationic current in rat ventricular cells. Br J Pharmacol 113:982–986
Vahlensieck U, Bokník P, Gombosová I et al (1999) Inotropic effects of diadenosine tetraphosphate (AP4A) in human and animal cardiac preparations. J Pharmacol Exp Ther 288:805–813
Balogh J, Wihlborg AK, Isackson H et al (2005) Phospholipase C and cAMP-dependent positive inotropic effects of ATP in mouse cardiomyocytes via P2Y11-like receptors. J Mol Cell Cardiol 39:223–230
Flitney FW, Singh J (1980) Inotropic responses of the frog ventricle to adenosine triphosphate and related changes in endogenous cyclic nucleotides. J Physiol 304:21–42
Puceat M, Bony C, Jaconi M et al (1998) Specific activation of adenylyl cyclase V by a purinergic agonist. FEBS Lett 431:189–194
Communi D, Govaerts C, Parmentier M et al (1997) Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem 272:31969–31973
Communi D, Robaye B, Boeynaems JM (1999) Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol 128:1199–1206
Zambon AC, Hughes RJ, Meszaros JG et al (2000) P2Y2 receptor of MDCK cells: cloning, expression, and cell-specific signaling. Am J Physiol Renal Physiol 279:F1045–F1052
Hou M, Malmsjö M, Möller S et al (1999) Increase in cardiac P2X1-and P2Y2-receptor mRNA levels in congestive heart failure. Life Sci 65:1195–1206
Froldi G, Varani K, Chinellato A et al (1997) P2X-purinoceptors in the heart: actions of ATP and UTP. Life Sci 60:1419–1430
Froldi G, Ragazzi E, Caparrotta L (2001) Do ATP and UTP involve cGMP in positive inotropism on rat atria? Comp Biochem Physiol C Toxicol Pharmacol 128:265–274
Erlinge D, Harnek J, van Heusden C et al (2005) Uridine triphosphate (UTP) is released during cardiac ischemia. Int J Cardiol 100:427–433
Borna C, Lazarowski E, van Heusden C et al (2005) Resistance to aspirin is increased by ST-elevation myocardial infarction and correlates with adenosine diphosphate levels. Thromb J 3:10
Kittel A, Kiss AL, Müllner N et al (2005) Expression of NTPDase1 and caveolins in human cardiovascular disease. Histochem Cell Biol 124:51–59
Gunduz D, Kasseckert SA, Härtel FV et al (2006) Accumulation of extracellular ATP protects against acute reperfusion injury in rat heart endothelial cells. Cardiovasc Res 71:764–773
Pham TM, Morris JB, Arthur JF et al (2003) UTP but not ATP causes hypertrophic growth in neonatal rat cardiomyocytes. J Mol Cell Cardiol 35:287–292
Zheng JS, Boluyt MO, O’Neill L et al (1994) Extracellular ATP induces immediate-early gene expression but not cellular hypertrophy in neonatal cardiac myocytes. Circ Res 74:1034–1041
Morris JB, Pham TM, Kenney B et al (2004) UTP transactivates epidermal growth factor receptors and promotes cardiomyocyte hypertrophy despite inhibiting transcription of the hypertrophic marker gene, atrial natriuretic peptide. J Biol Chem 279:8740–8746
Yitzhaki S, Shneyvays V, Jacobson KA et al (2005) Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress. Biochem Pharmacol 69:1215–1223
Yitzhaki S, Shainberg A, Cheporko Y et al (2006) Uridine-5’-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct. Biochem Pharmacol 72:949–955
Saini HK, Shao Q, Musat S et al (2005) Imidapril treatment improves the attenuated inotropic and intracellular calcium responses to ATP in heart failure due to myocardial infarction. Br J Pharmacol 144:202–211
Banfi C, Ferrario S, De Vincenti O et al (2005) P2 receptors in human heart: upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFα and potential role in myocardial cell death. J Mol Cell Cardiol 39:929–939
Malmsjö M, Bergdahl A, Möller S et al (1999) Congestive heart failure induces downregulation of P2X1-receptors in resistance arteries. Cardiovasc Res 43:219–227
Yang A, Sonin D, Jones L et al (2004) A beneficial role of cardiac P2X4 receptors in heart failure: rescue of the calsequestrin overexpression model of cardiomyopathy. Am J Physiol Heart Circ Physiol 287:H1096–H1103
Horackova M, Huang MH, Armour JA (1994) Purinergic modulation of adult guinea pig cardiomyocytes in long term cultures and co-cultures with extracardiac or intrinsic cardiac neurones. Cardiovasc Res 28:673–679
Jiang L, Bardini M, Keogh A et al (2005) P2X1 receptors are closely associated with connexin 43 in human ventricular myocardium. Int J Cardiol 98:291–297
Gaarder A, Jonsen J, Laland S et al (1961) Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 192:531–532
Daniel JL, Dangelmaier C, Jin J et al (1998) Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem 273:2024–2029
Jin J, Kunapuli SP (1998) Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA 95:8070–8074
Jin J, Daniel JL, Kunapuli SP (1998) Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem 273:2030–2034
Kunapuli SP, Dorsam RT, Kim S et al (2003) Platelet purinergic receptors. Curr Opin Pharmacol 3:175–180
Gachet C, Hechler B (2005) The platelet P2 receptors in thrombosis. Semin Thromb Hemost 31:162–167
Foster CJ, Prosser DM, Agans JM et al (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107:1591–1598
Fabre JE, Nguyen M, Latour A et al (1999) Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med 5:1199–1202
Leon C, Alex M, Klocke A et al (2004) Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood 103:594–600
Gachet C (2005) The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther 108:180–192
Leon C, Hechler B, Freund M et al (1999) Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest 104:1731–1737
Mahaut-Smith MP, Tolhurst G, Evans RJ (2004) Emerging roles for P2X1 receptors in platelet activation. Platelets 15:131–144
Birk AV, Leno E, Robertson HD et al (2003) Interaction between ATP and catecholamines in stimulation of platelet aggregation. Am J Physiol Heart Circ Physiol 284:H619–H625
Sévigny J, Sundberg C, Braun N et al (2002) Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood 99:2801–2809
Robson SC, Kaczmarek E, Siegel JB et al (1997) Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185:153–163
Koyamada N, Miyatake T, Candinas D et al (1996) Apyrase administration prolongs discordant xenograft survival. Transplantation 62:1739–1743
Imai M, Takigami K, Guckelberger O et al (2000) Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation 70:864–870
Mizumoto N, Kumamoto T, Robson SC et al (2002) CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med 8:358–365
Pearson JD (1993) The control of production and release of haemostatic factors in the endothelial cell. Baillieres Clin Haematol 6:629–651
Bouchie JL, Chen HC, Carney R et al (2000) P2Y receptor regulation of PAI-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 20:866–873
CAPRIE Steering Committee (1996) A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 348:1329–1339
Mehta SR, Yusuf S, Peters RJ et al (2001) Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358:527–533
Payne DA, Jones CI, Hayes PD et al (2004) Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation 109:1476–1481
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11302-007-9092-9
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Erlinge, D., Burnstock, G. P2 receptors in cardiovascular regulation and disease. Purinergic Signalling 4, 1–20 (2008). https://doi.org/10.1007/s11302-007-9078-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9078-7