Abstract
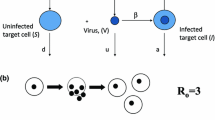
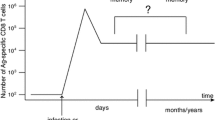
A major question in immunology is what role antigen load plays in determining the size of the CD8 immune response. Is the amount of antigen important during recruitment, proliferation, and/or memory formation? Animal studies have shown that antigen is only strictly required early during activation of T cells, but the importance of antigen at later timepoints is unclear. Using data from 24 volunteers infected with the yellow fever vaccine virus (YFV), we analyzed the dependence of T cell proliferation upon viral load. We found that volunteers with high viral load initially have greater T cell responses, but by 28 days post-vaccination those with lower viral load are able to ‘catch-up.’ Using differential equation modeling we show that this pattern is consistent with viral load only affecting recruitment (i.e., programmed proliferation) as opposed to affecting recruitment and proliferation (i.e., antigen-dependent proliferation). A quantitative understanding of the dependence of T cell dynamics on antigen load will be of use to modelers studying not only vaccination, but also cancer immunology and autoimmune disorders.






Similar content being viewed by others
References
Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, MM J, Ahmed R (2009) The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T-cell response. J Immunol 183(12):7919–7930
Akondy RS, Johnson PL, Nakaya HI, Edupuganti S, Mulligan MJ, Lawson B, Miller JD, Pulendran B, Antia R, Ahmed R (2015) Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. PNAS 112(10):3050–3055
Alanio C, Lemaitre F, Law HK, Hasan M, Albert ML (2010) Enumeration of human antigen-specific naive CD8+ T-cells reveals conserved precursor frequencies. Blood 115(18):3718–3725
Antia R, Koella JC (1994) A model of non-specific immunity. J Theor Biol 168(2):141–150
Antia R, Levin BR, May RM (1994) Within-host population dynamics and the evolution and maintenance of microparasite virulence. Am Nat 144:457–472
Antia R, Bergstrom CT, Pilyugin SS, Kaech SM, Ahmed R (2003) Models of CD8+ responses: 1. What is the antigen-independent proliferation program. J Theor Biol 221(4):585–598
Antia R, Ganusov VV, Ahmed R (2005) The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol 5(2):101–111
Badovinac VP, Porter BB, Harty JT (2002) Programmed contraction of CD8+ T cells after infection. Nat Immunol 3(7):619–626
Badovinac VP, Haring JS, Harty JT (2007) Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity 26(6):827–841
Ciupe M, Bivort B, Bortz D, Nelson P (2006) Estimating kinetic parameters from hiv primary infection data through the eyes of three different mathematical models. Math Biosci 200(1):1–27
Davis MM (2008) A prescription for human immunology. Immunity 29(6):835–838
De Boer RJ, Perelson AS (1995) Towards a general function describing t cell proliferation. J Theor Biol 175(4):567–576
De Boer RJ, Perelson AS (1998) Target cell limited and immune control models of hiv infection: a comparison. J Theor Biol 190(3):201–214
De Boer RJ, Oprea M, Antia R, Murali-Krishna K, Ahmed R, Perelson AS (2001) Recruitment times, proliferation, and apoptosis rates during the CD8+ T-cell response to lymphocytic choriomeningitis virus. J Virol 75(22):10,663–10,669
De Boer RJ, Homann D, Perelson AS (2003) Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol 171(8):3928–3935
De Boer RJ, Ganusov VV, Milutinović D, Hodgkin PD, Perelson AS (2006) Estimating lymphocyte division and death rates from CFSE data. Bull Math Biol 68(5):1011–1031
de Pillis LG, Radunskaya AE, Wiseman CL (2005) A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res 65(17):7950–7958
Edupuganti S, Eidex RB, Keyserling H, Akondy RS, Lanciotti R, Orenstein W, del Rio C, Pan Y, Querec T, Lipman H, Barrett A, Ahmed R, Teuwen D, Cetron M, Mulligan MJ (2013) A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity. Am J Trop Med Hyg 88(1):172–177
Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP (2007) Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med 204(2):299–309
Ganusov VV, Milutinović D, De Boer RJ (2007) IL-2 regulates expansion of CD4+ T cell populations by affecting cell death: insights from modeling CFSE data. J Immunol 179(2):950–957
Jaberi-Douraki M, Pietropaolo M, Khadra A (2014) Predictive models of type 1 diabetes progression: understanding T cell cycles and their implications on autoantibody release. PLoS ONE 9(4):e93,326
Kaech SM, Ahmed R (2001) Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol 2(5):415–422
Khadra A, Santamaria P, Edelstein-Keshet L (2009) The role of low avidity T cells in the protection against type 1 diabetes: a modeling investigation. J Theor Biol 256(1):126–141
Kim PS, Levy D, Lee PP (2009) Modeling and simulation of the immune system as a self-regulating network. Methods Enzymol 467:79–109
Mahaffy JM, Edelstein-Keshet L (2007) Modeling cyclic waves of circulating T cells in autoimmune diabetes. SIAM J Appl Math 67(4):915–937
Marchingo JM, Kan A, Sutherland RM, Duffy KR, Wellard CJ, Belz GT, Lew AM, Dowling MR, Heinzel S, Hodgkin PD (2014) Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 346(6213):1123–1127
Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG (2000) Early programming of T cell populations responding to bacterial infection. J Immunol 165(12):6833–6839
Monath TP (2005) Yellow fever vaccine. Expert Rev Vaccines 4(4):553–574
Moore H, Li NK (2004) A mathematical model for chronic myelogenous leukemia (CML) and T cell interaction. J Theor Biol 227(4):513–523
Moore J, Ahmed H, Jia J, Akondy R, Ahmed R, Antia R (2018) What controls the acute viral infection following yellow fever vaccination? Bull Math Biol 80(1):46–63
Nowak MA, Bangham CR (1996) Population dynamics of immune responses to persistent viruses. Science 272(5258):74–79
Perelson AS (2002) Modelling viral and immune system dynamics. Nat Rev Immunol 2(1):28–36
Smith J, Martin L (1973) Do cells cycle? Proc Natl Acad Sci 70(4):1263–1267
Terry E, Marvel J, Arpin C, Gandrillon O, Crauste F (2012) Mathematical model of the primary CD8 T cell immune response: stability analysis of a nonlinear age-structured system. JMB 65(2):263–291
van Stipdonk MJ, Lemmens EE, Schoenberger SP (2001) Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol 2(5):423–429
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12(6):492
Williams MA, Bevan MJ (2004) Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol 173(11):6694–6702
Willis RA, Kappler JW, Marrack PC (2006) CD8 T cell competition for dendritic cells in vivo is an early event in activation. PNAS 103(32):12,063–12,068
Wilson S, Levy D (2012) A mathematical model of the enhancement of tumor vaccine efficacy by immunotherapy. Bull Math Biol 74(7):1485–1500
Zarnitsyna VI, Handel A, McMaster SR, Hayward SL, Kohlmeier JE, Antia R (2016) Mathematical model reveals the role of memory CD8 T cell populations in recall responses to influenza. Front Immunol 7(3):165
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by four NIH Grants NIH U54GM111274, NIH R01AI110720 (to R. Antia), NIH U19AI11789102 (to R. Antia and R. Ahmed), and U19AI057266 (to R. Ahmed).
Rights and permissions
About this article
Cite this article
Moore, J.R., Ahmed, H., McGuire, D. et al. Dependence of CD8 T Cell Response upon Antigen Load During Primary Infection. Bull Math Biol 81, 2553–2568 (2019). https://doi.org/10.1007/s11538-019-00618-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-019-00618-9