Abstract
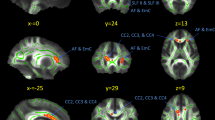
Alcoholism can lead to a complex mixture of cognitive and emotional deficits associated with abnormalities in fronto-cortico-striatal-limbic brain circuitries. Given the broad variety of neurobehavioral symptoms, one would also expect alterations of postrolandic neocortical systems. Thus, we used diffusion tensor imaging (DTI) to study the integrity of the middle longitudinal fascicle (MdLF), a major postrolandic association white matter tract that extends from the superior temporal gyrus to the parietal and occipital lobes, in individuals with a history of chronic alcohol abuse. DTI data were acquired on a 3 Tesla scanner in 30 abstinent alcoholics (AL; 9 men) and 25 nonalcoholic controls (NC; 8 men). The MdLF was determined using DTI-based tractography. Volume of the tract, fractional anisotropy (FA), radial (RD), and axial (AD) diffusivity, were compared between AL and NC, with sex and hemispheric laterality as independent variables. The association of DTI measures with neuropsychological performance was evaluated. Men showed bilateral reduction of MdLF volume and abnormal diffusion measurements of the left MdLF. Analyses also indicated that the left MdLF diffusion measurements in AL men were negatively associated with Verbal IQ and verbal fluency test scores. Abstinent alcoholic men display macrostructural abnormalities in the MdLF bilaterally, indicating an overall white matter deficit. Additionally, microstructural deficits of the left MdLF suggest more specific alterations associated with verbal skills in men.




Similar content being viewed by others
References
Acheson, A., Wijtenburg, S. A., Rowland, L. M., Bray, B. C., Gaston, F., Mathias, C. W., et al. (2014). Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Human Brain Mapping, 35(12), 5877–5887. doi:10.1002/hbm.22591.
Alhassoon, O. M., Sorg, S. F., Taylor, M. J., Stephan, R. A., Schweinsburg, B. C., Stricker, N. H., et al. (2012). Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcoholism, Clinical and Experimental Research, 36(11), 1922–1931. doi:10.1111/j.1530-0277.2012.01808.x.
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association.
Arnett, J. A., & Seth, S. L. (1995). Effect of physical layout in performance of the trail making test. Psychological Assessment, 7(2), 220–221.
Barbas, H. (2000). Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin, 32(5), 319–330.
Barbas, H., Saha, S., Rempel-Clower, N., & Ghashghaei, T. (2003). Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience, 4(1), 25–37.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219.
Benton, A. L. (1969). Development of a multilingual aphasia battery. Progress and problems. Journal of the Neurological Sciences, 9(1), 39–48.
Blum, K., Braverman, E. R., Holder, J. M., Lubar, J. F., Monastra, V. J., Miller, D., et al. (2000). Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs, 32(Suppl, i-iv), 1–112.
Bowirrat, A., & Oscar-Berman, M. (2005). Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 132B(1), 29–37. doi:10.1002/ajmg.b.30080.
Bravo, F., Gual, A., Lligona, A., & Colom, J. (2013). Gender differences in the long-term outcome of alcohol dependence treatments: an analysis of twenty-year prospective follow up. Drug and Alcohol Review, 32(4), 381–388. doi:10.1111/dar.12023.
Cahalan, D., Cisin, I., & Crossley, H. (1969). American drinking practices: A national study of drinking behavior and attitudes (monograph no. 6). New Brunswick, NJ: Rutgers Center for Alcohol Studies.
Catani, M., Allin, M. P., Husain, M., Pugliese, L., Mesulam, M. M., Murray, R. M., & Jones, D. K. (2007). Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America, 104(43), 17163–17168.
Caviness Jr., V. S., Lange, N. T., Makris, N., Herbert, M. R., & Kennedy, D. N. (1999). MRI-based brain volumetrics: emergence of a developmental brain science. Brain Dev, 21(5), 289–295.
Chiarello, C., Welcome, S. E., Halderman, L. K., Towler, S., Julagay, J., Otto, R., & Leonard, C. M. (2009). A large-scale investigation of lateralization in cortical anatomy and word reading: are there sex differences? Neuropsychology, 23(2), 210–222. doi:10.1037/a0014265.
Church, M. W., & Abel, E. L. (1998). Fetal alcohol syndrome. Hearing, speech, language, and vestibular disorders. Obstetrics and Gynecology Clinics of North America, 25(1), 85–97.
Clements, A. M., Rimrodt, S. L., Abel, J. R., Blankner, J. G., Mostofsky, S. H., Pekar, J. J., et al. (2006). Sex differences in cerebral laterality of language and visuospatial processing. Brain and Language, 98(2), 150–158.
Danielsson, A. K., Romelsjo, A., & Tengstrom, A. (2011). Heavy episodic drinking in early adolescence: gender-specific risk and protective factors. Substance Use & Misuse, 46(5), 633–643. doi:10.3109/10826084.2010.528120.
Fitzpatrick, L. E., Jackson, M., & Crowe, S. F. (2008). The relationship between alcoholic cerebellar degeneration and cognitive and emotional functioning. Neuroscience and Biobehavioral Reviews, 32(3), 466–485.
Foisy, M. L., Kornreich, C., Petiau, C., Parez, A., Hanak, C., Verbanck, P., et al. (2007). Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatry Res: Neuroimaging, 150(1), 33–41.
Fortier, C. B., Leritz, E. C., Salat, D. H., Lindemer, E., Maksimovskiy, A. L., Shepel, J., et al. (2014). Widespread effects of alcohol on white matter microstructure. Alcoholism, Clinical and Experimental Research, 38(12), 2925–2933. doi:10.1111/acer.12568.
GraphPadSoftware. (2014). GraphPad Prism version 6.00 for Windows. San Diego California USA GraphPadSoftware. Retrieved from www.graphpad.com.
Hahn, C., Pogun, S., & Gunturkun, O. (2010). Smoking modulates language lateralization in a sex-specific way. Neuropsychologia, 48(14), 3993–4002. doi:10.1016/j.neuropsychologia.2010.10.014.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera, F., & Weinberger, D. R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53(6), 494–501.
Harper, C., Dixon, G., Sheedy, D., & Garrick, T. (2003). Neuropathological alterations in alcoholic brains. Studies arising from the new South Wales tissue resource Centre. Progress Neuro-psychopharmacol Biol Psychiatry, 27(6), 951–961. doi:10.1016/S0278-5846(03)00155-6.
Harris, G. J., Jaffin, S. K., Hodge, S. M., Kennedy, D., Caviness, V. S., Marinkovic, K., et al. (2008). Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism, Clinical and Experimental Research, 32(6), 1001–1013. doi:10.1111/j.1530-0277.2008.00661.x.
Healey, J. M., Waldstein, S., & Goodglass, H. (1985). Sex differences in the lateralization of language discrimination vs language production. Neuropsychologia, 23(6), 777–789.
IBMCorp. (2013). IBM SPSS statistics for windows, version 22.0. Armonk, NY: IBMCorp.
Ito, R., Mori, S., & Melhem, E. R. (2002). Diffusion tensor brain imaging and tractography. Neuroimaging Clinics of North America, 12(1), 1–19.
Jenkinson, M., Bannister, P., Brady, J. M., & Smith, S. M. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. doi:10.1016/j.neuroimage.2012.06.081.
Kansaku, K., & Kitazawa, S. (2001). Imaging studies on sex differences in the lateralization of language. Neuroscience Research, 41(4), 333–337.
Kansaku, K., Yamaura, A., & Kitazawa, S. (2000). Sex differences in lateralization revealed in the posterior language areas. Cerebral Cortex, 10(9), 866–872.
Kong, L. M., Zheng, W. B., Lian, G. P., & Zhang, H. D. (2012). Acute effects of alcohol on the human brain: diffusion tensor imaging study. American Journal of Neuroradiology, 33(5), 928–934. doi:10.3174/ajnr.A2873.
Koob, G. F. (2014). Neurocircuitry of alcohol addiction: Synthesis from animal models. In A. Pfefferbaum & E. V. Sullivan (Eds.), Handbook of clinical neurology: Alcohol and the nervous system (pp. 33–54). Edinburgh: Elsevier. doi:10.1016/B978-0-444-62619-6.00003-3.
Korkman, M., Hilakivi-Clarke, L. A., Autti-Ramo, I., Fellman, V., & Granstrom, M. L. (1994). Cognitive impairments at two years of age after prenatal alcohol exposure or perinatal asphyxia. Neuropediatrics, 25(2), 101–105. doi:10.1055/s-2008-1071594.
Lai, X. P., Yu, X. J., Qian, H., Wei, L., Lv, J. Y., & Xu, X. H. (2013). Chronic alcoholism-mediated impairment in the medulla oblongata: a mechanism of alcohol-related mortality in traumatic brain injury? Cell Biochemistry and Biophysics, 67(3), 1049–1057. doi:10.1007/s12013-013-9603-y.
Lancaster, J. L., Woldorff, M. G., Parsons, L. M., Liotti, M., Freitas, C. S., Rainey, L., et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. doi:10.1002/1097-0193(200007)10:3<120::AID-.
MacVane, J., Butters, N., Montgomery, K., & Farber, J. (1982). Cognitive functioning in men social drinkers; a replication study. Journal of Studies on Alcohol, 43(1), 81–95.
Maiya, R. P., & Messing, R. O. (2014). Peripheral systems: Neuropathy. In A. Pfefferbaum & E. V. Sullivan (Eds.), Handbook of clinical neurology: Alcohol and the nervous system (pp. 513–525). Edinburgh: Elsevier. doi:10.1016/B978-0-444-62619-6.00029-X.
Makris, N. (1999). Delineation of human association fiber pathways using histologic and magnetic resonance methodologies. Ph.D. dissertation, Boston University, Boston MA.
Makris, N., Gasic, G. P., Kennedy, D. N., Hodge, S. M., Kaiser, J. R., Lee, M. J., & Breiter, H. C. (2008a). Cortical thickness abnormalities in cocaine addiction--a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron, 60(1), 174–188. doi:10.1016/j.neuron.2008.08.011.
Makris, N., Oscar-Berman, M., Jaffin, S. K., Hodge, S. M., Kennedy, D. N., Caviness, V. S., & Harris, G. J. (2008b). Decreased volume of the brain reward system in alcoholism. Biological Psychiatry, 64(3), 192–202. doi:10.1016/j.biopsych.2008.01.018.
Makris, N., Papadimitriou, G. M., Kaiser, J. R., Sorg, S., Kennedy, D. N., & Pandya, D. N. (2009). Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex, 19(4), 777–785. doi:10.1093/cercor/bhn124.
Makris, N., Preti, M. G., Asami, T., Pelavin, P., Campbell, B., Papadimitriou, G. M., & Kubicki, M. (2013a). Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Structure & Function, 218(4), 951–968. doi:10.1007/s00429-012-0441-2.
Makris, N., Preti, M. G., Wassermann, D., Rathi, Y., Papadimitriou, G. M., Yergatian, C., & Kubicki, M. (2013b). Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging and Behavior, 7(3), 335–352. doi:10.1007/s11682-013-9235-2.
Maldonado, I. L., de Champfleur, N. M., Velut, S., Destrieux, C., Zemmoura, I., & Duffau, H. (2013). Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. Journal of Anatomy, 223(1), 38–45. doi:10.1111/joa.12055.
Marinkovic, K., Oscar-Berman, M., Urban, T., O’Reilly, C. E., Howard, J. A., Sawyer, K., & Harris, G. J. (2009). Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism, Clinical and Experimental Research, 33(11), 1880–1892. doi:10.1111/j.1530-0277.2009.01026.x.
Martino, J., da Silva-Freitas, R., Caballero, H., Marco de Lucas, E., Garcia-Porrero, J. A., & Vazquez-Barquero, A. (2013a). Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery, 72(1 Suppl Operative), 87–97 discussion 97-88. doi:10.1227/NEU.0b013e318274294b.
Martino, J., De Witt Hamer, P. C., Berger, M. S., Lawton, M. T., Arnold, C. M., de Lucas, E. M., & Duffau, H. (2013b). Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Structure & Function, 218(1), 105–121. doi:10.1007/s00429-012-0386-5.
Melhem, E. R., Mori, S., Mukundan, G., Kraut, M. A., Pomper, M. G., & van Zijl, P. C. (2002). Diffusion tensor MR imaging of the brain and white matter tractography. AJR. American Journal of Roentgenology, 178(1), 3–16. doi:10.2214/ajr.178.1.1780003.
Mellion, M. L., Nguyen, V., Tong, M., Gilchrist, J., & De La Monte, S. (2013). Experimental model of alcohol-related peripheral neuropathy. Muscle & Nerve, 48(2), 204–211. doi:10.1002/mus.23744.
Mellion, M. L., Silbermann, E., Gilchrist, J. M., Machan, J. T., Leggio, L., & de la Monte, S. (2014). Small-fiber degeneration in alcohol-related peripheral neuropathy. Alcoholism, Clinical and Experimental Research, 38(7), 1965–1972. doi:10.1111/acer.12470.
Menjot de Champfleur, N., Lima Maldonado, I., Moritz-Gasser, S., Machi, P., Le Bars, E., Bonafe, A., & Duffau, H. (2013). Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. European Journal of Radiology, 82(1), 151–157. doi:10.1016/j.ejrad.2012.05.034.
Mlinarics, R., Kelemen, O., Sefcsik, T., & Nemeth, D. (2009). Cognitive impairment in patients with alcoholism after long-term abstinence. Neuropsychopharmacol Hung, 11(3), 135–139. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20128392
Mori, S., Crain, B. J., Chacko, V. P., & van Zijl, P. C. (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45(2), 265–269.
Moselhy, H. F., Georgiou, G., & Kahn, A. (2001). Frontal lobe changes in alcoholism: a review of the literature. Alcohol and Alcoholism, 36(5), 357–368.
Nguyen, V. A., Le, T., Tong, M., Mellion, M., Gilchrist, J., & de la Monte, S. M. (2012). Experimental alcohol-related peripheral neuropathy: role of insulin/IGF resistance. Nutrients, 4(8), 1042–1057. doi:10.3390/nu4081042.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Robertson, E. R., Chopra, S., Gabrieli, J. D. E., & Gross, J. J. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–499.
Oscar-Berman, M., & Bowirrat, A. (2005). Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Disease and Treatment, 1(3), 1–19.
Oscar-Berman, M., & Marinkovic, K. (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257.
Oscar-Berman, M., & Song, J. (2011). Brain volumetric measures in alcoholics: a comparison of two segmentation methods. Neuropsychiatric Disease and Treatment, 7, 65–75. doi:10.2147/NDT.S13405.
Oscar-Berman, M., Hancock, M., Mildworf, B., & Hutner, N. (1990). Emotional perception and memory in alcoholism and aging. Alcoholism, Clinical and Experimental Research, 14(3), 383–393.
Oscar-Berman, M., Valmas, M. M., Sawyer, K. S., Ruiz, S. M., Luhar, R. B., & Gravitz, Z. R. (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. In A. Pfefferbaum & E. V. Sullivan (Eds.), Handbook of clinical neurology: Alcohol and the nervous system (pp. 183–210). Edinburgh: Elsevier. doi:10.1016/B978-0-444-62619-6.00012-4.
Pasternak, O., Sochen, N., Gur, Y., Intrator, N., & Assaf, Y. (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine, 62, 717–730.
Pfefferbaum, A., Adalsteinsson, E., & Sullivan, E. V. (2006). Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry, 59(4), 364–372.
Piccirilli, M., D'Alessandro, P., Finali, G., Maiotti, M., Piccinin, G. L., & Agostini, L. (1988). Individual differences in cerebral organization: influence of sex and familial sinistrality in the language lateralization of strongly right-handed subjects. Functional Neurology, 3(3), 285–299.
Pitel, A. L., Witkowski, T., Vabret, F., Guillery-Girard, B., Desgranges, B., Eustache, F., & Beaunieux, H. (2007). Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcoholism, Clinical and Experimental Research, 31(2), 238–248.
Poser, C. M. (1973). Demyelination in the central nervous system in chronic alcoholism: central pontine myelinolysis and Marchiafava-Bignami's disease. Annals of the New York Academy of Sciences, 215, 373–381.
Prendergast, M. L., Messina, N. P., Hall, E. A., & Warda, U. S. (2011). The relative effectiveness of women-only and mixed-gender treatment for substance-abusing women. Journal of Substance Abuse Treatment, 40(4), 336–348. doi:10.1016/j.jsat.2010.12.001.
Rasmussen, C., Tamana, S., Baugh, L., Andrew, G., Tough, S., & Zwaigenbaum, L. (2013). Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychology, 19(4), 337–349. doi:10.1080/09297049.2012.658768.
Rathi, Y., Kubicki, M., Bouix, S., Westin, C. F., Goldstein, J., Seidman, L., Mesholam-Gately, R., McCarley, R. W., & Shenton, M. E. (2011). Statistical analysis of fiber bundles using multi-tensor tractography: application to first-episode schizophrenia. Magnetic Resonance Imaging, 29, 507–515.
Robins, L., Helzer, J., Cottler, L., & Goldring, E. (1989). NIMH diagnostic interview schedule: Version III revised (DIS-III_R). St Louis, MO: Washington University Louis.
Ruiz, S. M., Oscar-Berman, M., Sawyer, K. S., Valmas, M. M., Urban, T., & Harris, G. J. (2013). Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, Clinical and Experimental Research, 37(1), 110–122. doi:10.1111/j.1530-0277.2012.01862.x.
Samantaray, S., Knaryan, V. H., Patel, K. S., Mulholland, P. J., Becker, H. C., & Banik, N. L. (2015). Chronic intermittent ethanol induced axon and myelin degeneration is attenuated by calpain inhibition. Brain Res.
Sandu, A. L., Specht, K., Beneventi, H., Lundervold, A., & Hugdahl, K. (2008). Sex-differences in grey-white matter structure in normal-reading and dyslexic adolescents. Neuroscience Letters, 438(1), 80–84. doi:10.1016/j.neulet.2008.04.022.
Sawyer, K. S., Papadimitriou, G., Ruiz, S. M., Makris, N., Oscar-Berman, M., & Harris, G. J. (2015). Gender dimorphism of white matter integrity assessed by diffusion tensor magnetic resonance imaging in abstinent alcoholic men and women. Paper presented at the addiction health services research (AHSR) conference, Boston, MA, USA.
Schmidt, P. J., Berlin, K. L., Danaceau, M. A., Neeren, A., Haq, N. A., Roca, C. A., & Rubinow, D. R. (2004). The effects of pharmacologically induced hypogonadism on mood in healthy men. Archives of General Psychiatry, 61(10), 997–1004.
Schulte, T., Muller-Oehring, E. M., Pfefferbaum, A., & Sullivan, E. V. (2010). Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues in Clinical Neuroscience, 12(4), 554–560.
Segobin, S., Ritz, L., Lannuzel, C., Boudehent, C., Vabret, F., Eustache, F., & Pitel, A. L. (2015). Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Human Brain Mapping, 36(7), 2795–2808. doi:10.1002/hbm.22808.
Sher, L. (2007). Functional magnetic resonance imaging in studies of the neurobiology of suicidal behavior in adolescents with alcohol use disorders. International Journal of Adolescent Medicine and Health, 19(1), 11–18.
Smith, M. E., & Oscar-Berman, M. (1992). Resource-limited information processing in alcoholism. Journal of Studies on Alcohol, 53(5), 514–518.
Sommer, I. E., Aleman, A., Somers, M., Boks, M. P., & Kahn, R. S. (2008). Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Research, 1206, 76–88. doi:10.1016/j.brainres.2008.01.003.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20(3), 1714–1722.
Squeglia, L. M., Sorg, S. F., Schweinsburg, A. D., Wetherill, R. R., Pulido, C., & Tapert, S. F. (2012). Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology, 220(3), 529–539. doi:10.1007/s00213-011-2500-4.
Squeglia, L. M., Sorg, S. F., Jacobus, J., Brumback, T., Taylor, C. T., & Tapert, S. F. (2015). Structural connectivity of neural reward networks in youth at risk for substance use disorders. Psychopharmacology, 232(13), 2217–2226. doi:10.1007/s00213-014-3857-y.
Tombaugh, T. N. (2004). Trail making test a and B: normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. doi:10.1016/S0887-6177(03)00039-8.
Vikingstad, E. M., George, K. P., Johnson, A. F., & Cao, Y. (2000). Cortical language lateralization in right handed normal subjects using functional magnetic resonance imaging. Journal of the Neurological Sciences, 175(1), 17–27.
Wang, R., Benner, T., Sorensen, A. G., & Wedeen, V. J. (2007). Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Paper presented at the ISMRM, Berlin, Germany.
Wang, Y., Fernandez-Miranda, J. C., Verstynen, T., Pathak, S., Schneider, W., & Yeh, F. C. (2013). Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cerebral Cortex, 23(10), 2347–2356. doi:10.1093/cercor/bhs225.
Wechsler, D. (1997a). Wechsler adult intelligence scale (3 ed.). San Antonio, Texas: Psychological Corporation.
Wechsler, D. (1997b). Wechsler memory scale administration and scoring manual. San Antonio, Texas: Psychological Corporation.
Wyper, K. R., & Rasmussen, C. R. (2011). Language impairments in children with fetal alcohol spectrum disorders. Journal of Population Therapeutics and Clinical Pharmacology, 18(2), e364–e376.
Yu, V. Y., MacDonald, M. J., Oh, A., Hua, G. N., De Nil, L. F., & Pang, E. W. (2014). Age-related sex differences in language lateralization: a magnetoencephalography study in children. Developmental Psychology, 50(9), 2276–2284. doi:10.1037/a0037470.
Zahr, N. M. (2014). Structural and microstructral imaging of the brain in alcohol use disorders. Handbook of Clinical Neurology, 125, 275–290. doi:10.1016/B978-0-444-62619-6.00017-3.
Zorlu, N., Karavul Ucman, T., Gelal, F., Colak Kalayci, C., Polat, S., Saricicek, A., & Gulseren, S. (2014). Abnormal white matter integrity in long-term abstinent alcohol dependent patients. Psychiatry Research, 224(1), 42–48. doi:10.1016/j.pscychresns.2014.07.006.
Acknowledgments
The authors thank Mary M. Valmas, Anne-Mette Guldberg, and Diane Merritt for recruitment assistance and neuropsychological testing, and Trinity Urban and Alan Poey for assistance with brain scan acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01AA07112 and K05AA00219, and by the US Department of Veterans Affairs Clinical Science Research and Development grant to Dr. Marlene Oscar Berman; by the National Institutes of Health grants P50MH080272 and R01 MH102377 to Dr. Marek Kubicki; by the National Institute of Neurological Disorders and Stroke grants R21NS077059 and R21NS079905, and by the National Institute on Aging grant R01AG042512 to Dr. Nikos Makris. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of interest
The Authors Johanna Seitz, Kayle S. Sawyer, Susan M. Ruiz, George Papadimitriou, Isaac Ng, Antoni Kubicki, Palig Mouradian, Marlene Oscar-Berman, Marek Kubicki, Gordon J. Harris, and Nikos Makris have declared that there are no conflicts of interest in relation to the subject of this study.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Marlene Oscar-Berman, Marek Kubicki, Gordon J. Harris and Nikos Makris contributed equally.
Rights and permissions
About this article
Cite this article
Seitz, J., Sawyer, K.S., Papadimitriou, G. et al. Alcoholism and sexual dimorphism in the middle longitudinal fascicle: a pilot study. Brain Imaging and Behavior 11, 1006–1017 (2017). https://doi.org/10.1007/s11682-016-9579-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9579-5