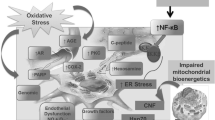
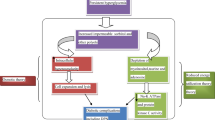
Abstract
Diabetic neuropathies (DNs) are one of the most prevalent chronic complications of diabetes and a major cause of disability, high mortality, and poor quality of life. Given the complex anatomy of the peripheral nervous system and types of fiber dysfunction, DNs have a wide spectrum of clinical manifestations. The treatment of DNs continues to be challenging, likely due to the complex pathogenesis that involves an array of systemic and cellular imbalances in glucose and lipids metabolism. These lead to the activation of various biochemical pathways, including increased oxidative/nitrosative stress, activation of the polyol and protein kinase C pathways, activation of polyADP ribosylation, and activation of genes involved in neuronal damage, cyclooxygenase-2 activation, endothelial dysfunction, altered Na+/K+-ATPase pump function, impaired C-peptide-related signaling pathways, endoplasmic reticulum stress, and low-grade inflammation. This review summarizes current evidence regarding the role of low-grade inflammation as a potential therapeutic target for DNs.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tesfaye S et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93.
American Diabetes Association, Standards of Medical Care in Diabetes. Diabetes Care. 2015;38:S4. doi:10.2337/dc15-S003.
Selvin E et al. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–25.
Imperatore G et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–20.
Pettitt DJ et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37(2):402–8.
Ang L et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. This is a omprehensive review of the evidence linking glucose control with prevention and reversal of neuropathy in type 1 and type 2 diabetes.
Spallone V et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–53.
Ramsey SD et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3):382–7.
Coppini DV et al. Showing neuropathy is related to increased mortality in diabetic patients—a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53(5):519–23.
Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–41.
Pop-Busui R et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–84.
Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res. 2012;5(4):463–78.
Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–51.
Pop-Busui R et al. Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications). J Am Coll Cardiol. 2013;61(4):447–54.
Lieb DC et al. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp Diabetes Res. 2012;2012:878760.
Chrisholm DJ. The Diabetes Control and Complications Trial (DCCT). A milestone in diabetes management. Med J Aust. 1993;159(11–12):721–3.
DCCT. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38(6):869–80.
Albers JW et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–6.
Pop-Busui R et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22):2886–93.
Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473.
Callaghan BC et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34. This paper sumarizes evidence on pathogenic treatments for diabetic neuropathy.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. This paper links low-grade inflammation with insulin resistance in type 2 diabetes.
Duncan BB et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–805.
Pradhan AD et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34.
Schmidt MI et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353(9165):1649–52.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. A seminal paper that demonstrates the effects of chronic inflammation on changes in metabolism and subsequent metabolic disorders.
Goldfine AB et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1(1):36–43.
Goldfine AB et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159(1):1–12.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. This paper unveiled links between inflammation and atherosclerosis and cardiovascular disease, a chronic complication of diabetes, which was a paradigm shift.
Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94(9):3171–82.
Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep. 2006;6(6):463–8.
Lopes-Virella MF et al. Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care. 2008;31(10):2006–12.
Wolkow PP et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19(4):789–97.
Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2015;38(6):954–62.
Krolewski AS et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37(1):226–34.
Bergmann M, Barnes PJ. Molecular biologic aspects of chronic inflammation reaction in bronchial asthma: incentive for new therapeutic concepts? Pneumologie. 1997;51(11):1071–8.
O'Shaughnessy TC et al. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852–7.
Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9(1):60–7.
Ye J et al. Alterations in cytokine regulation in aged epidermis: implications for permeability barrier homeostasis and inflammation. I. IL-1 gene family. Exp Dermatol. 2002;11(3):209–16.
Kellogg AP et al. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fibers loss in experimental diabetes. Diabetes. 2007;56(12):2997–3005.
Doupis J et al. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94(6):2157–63.
Hur J et al. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain. 2011;134(Pt 11):3222–35. This paper provided first evidence in humans regarding the role of inflammation and identified specfic gene expresion profiles including inflammatory genes associated with progression of human diabetic neuropahy.
Hur J et al. Identification of factors associated with sural nerve regeneration and degeneration in diabetic neuropathy. Diabetes Care. 2013;36(12):4043–9.
Pop-Busui R et al. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004;44(12):2368–74.
Barnes PJ, Adcock IM. NF-kappa B: a pivotal role in asthma and a new target for therapy. Trends Pharmacol Sci. 1997;18(2):46–50.
Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15(4):373–4.
Mattson MP et al. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6(1–2):69–81.
Wang Y et al. Enhanced inflammatory response via activation of NF-kappaB in acute experimental diabetic neuropathy subjected to ischemia-reperfusion injury. J Neurol Sci. 2006;247(1):47–52.
Purves T et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15(13):2508–14.
Cheng HT et al. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol Pain. 2010;6:28.
Andriambeloson E et al. Interleukin-6 attenuates the development of experimental diabetes-related neuropathy. Neuropathology. 2006;26(1):32–42.
Cotter MA et al. Effects of interleukin-6 treatment on neurovascular function, nerve perfusion and vascular endothelium in diabetic rats. Diabetes Obes Metab. 2010;12(8):689–99.
Asea A et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42.
Ma J et al. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Ther. 2014;348(2):281–92.
Ma J et al. Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chem Neurosci. 2015;6(9):1637–48.
Gruden G et al. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57(7):1966–70.
Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133–41.
Kim B et al. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011;152(10):3638–47.
Vincent AM et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–83.
Wiggin TD et al. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149(10):4928–37.
Vincent AM et al. Biology of diabetic neuropathy. Handb Clin Neurol. 2013;115:591–606.
Uceyler N et al. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69(1):42–9.
Duksal T et al. Role of inflammation in sensory neuropathy in prediabetes or diabetes. 2015. Acta Neurol Scand.
Herder C et al. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care. 2013;36(11):3663–70. Large population based study that reported associations between subclinical inflammation and diabetic neuropathy.
NIDDK N. Summary Report Charcot Workshop, co-sponsored by NIH's Office of Rare Diseases. 2008.
Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23(6):796–800.
Lavery LA et al. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26(5):1435–8.
Sinha S, Munichoodappa CS, Kozak GP. Neuro-arthropathy (Charcot joints) in diabetes mellitus (clinical study of 101 cases). Medicine (Baltimore). 1972;51(3):191–210.
Irie K et al. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech. 2002;58(2):85–90.
Weitzmann NM. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica. 2013;2013:125705. doi:10.1155/2013/125705.
Mabilleau G et al. Increased osteoclastic activity in acute Charcot's osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. 2008;51(6):1035–40.
Armstrong DG, Lavery LA. Monitoring healing of acute Charcot's arthropathy with infrared dermal thermometry. J Rehabil Res Dev. 1997;34(3):317–21.
Koeck FX et al. Marked loss of sympathetic nerve fibers in chronic Charcot foot of diabetic origin compared to ankle joint osteoarthritis. J Orthop Res. 2009;27(6):736–41.
Mabilleau G et al. Number of circulating CD14-positive cells and the serum levels of TNF-alpha are raised in acute charcot foot. Diabetes Care. 2011;34(3):e33.
Ndip A et al. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes. 2011;60(8):2187–96.
Armstrong DG et al. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14(5):357–63.
Petrova NL, Edmonds ME. Charcot neuro-osteoarthropathy-current standards. Diabetes Metab Res Rev. 2008;24 Suppl 1:S58–61.
Boulton AJ et al. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24.
Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5-year follow-up. Diabetes Care. 2001;24(1):78–83.
Izumi Y et al. Mortality of first-time amputees in diabetics: a 10-year observation. Diabetes Res Clin Pract. 2009;83(1):126–31.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–5S.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84.
Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14(7–8):485–92.
Weisberg SP et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Xu H et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43.
Loots MA et al. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111(5):850–7.
Pierce GF. Inflammation in nonhealing diabetic wounds: the space-time continuum does matter. Am J Pathol. 2001;159(2):399–403.
Wetzler C et al. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245–53.
Goren I et al. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127(9):2259–67.
Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276(5309):75–81.
Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–9.
Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338–46.
Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37 Suppl 1:S9–17.
Mantovani A et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Sutterwala FS et al. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185(11):1977–85.
Sutterwala FS et al. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188(1):217–22.
Martinez FO et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–61.
Roy S et al. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008;34(2):162–84.
Porcheray F et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–9.
Khanna S et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5(3):e9539.
Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15(11):599–607.
Sindrilaru A et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–97.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70.
Dou Y et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13(8):713–9.
Mukasa R et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32(5):616–27.
Pronk CJ et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1(4):428–42.
Fathke C et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–22.
Okuno Y et al. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117(19):5264–72.
Hirahara K et al. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134(3):235–45.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54.
Schlesinger Y et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39(2):232–6.
Guenther MG et al. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130(1):77–88.
Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396(6706):77–80.
Fleischman A et al. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31(2):289–94.
Aceves M et al. A new pharmacological effect of salicylates: inhibition of NFAT-dependent transcription. J Immunol. 2004;173(9):5721–9.
Lemay S, Lebedeva TV, Singh AK. Inhibition of cytokine gene expression by sodium salicylate in a macrophage cell line through an NF-kappaB-independent mechanism. Clin Diagn Lab Immunol. 1999;6(4):567–72.
Roman J et al. UR-1505, a new salicylate, blocks T cell activation through nuclear factor of activated T cells. Mol Pharmacol. 2007;72(2):269–79.
Tesfaye S et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50.
Wiggin TD et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–40.
Acknowledgments
The authors would like to thank Dr. Stacey Sakowski Jacoby for expert editorial assistance.
Funding was provided by the National Institutes of Health (1R01HL102334, 1R03 DK094499, and 1DP3DK094292 to R.P.B) and (1DP3DK094292, 1R24082841 to E.L.F.); Novo Nordisk Foundation (NNF14SA0006 to E.L.F.), Program for Neurology Research and Discovery; and the A. Alfred Taubman Medical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rodica Pop-Busui, Lynn Ang, Crystal Holmes, Katherine Gallagher, and Eva L. Feldman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Microvascular Complications—Neuropathy
Rights and permissions
About this article
Cite this article
Pop-Busui, R., Ang, L., Holmes, C. et al. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr Diab Rep 16, 29 (2016). https://doi.org/10.1007/s11892-016-0727-5
Published:
DOI: https://doi.org/10.1007/s11892-016-0727-5