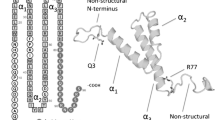
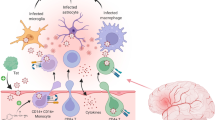
Abstract
HIV-1 is completely dependent upon the Env protein to enter cells. The virus typically replicates in activated CD4+ T cells due to viral entry requirements for the CCR5 coreceptor and for high surface levels of the CD4 receptor. This is the case for the transmitted virus and for most of the virus sampled in the blood. Over the course of infection, the env gene can evolve to encode a protein with altered receptor and coreceptor usage allowing the virus to enter alternative host cells. In about 50% of HIV-1 infections, the viral population undergoes coreceptor switching, usually late in disease, allowing the virus to use CXCR4 to enter a different subset of CD4+ T cells. Neurocognitive disorders occur in about 10% of infections, also usually late in disease, but caused (ultimately) by viral replication in the brain either in CD4+ T cells or macrophage and/or microglia. Expanded host range is significantly intertwined with pathogenesis. Identification and characterization of such HIV-1 variants may be useful for early detection which would allow intervention to reduce viral pathogenesis in these alternative cell types.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Leitner T, Kumar S, Albert J. Tempo and mode of nucleotide substitutions in gag and env gene fragments in human immunodeficiency virus type 1 populations with a known transmission history. J Virol. 1997;71:4761–70.
Asjo B, Morfeldt-Manson L, Albert J, et al. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;2:660–2.
Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700.
Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8.
Brenchley JM, Hill BJ, Ambrozak DR, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–8.
Sleasman JW, Aleixo LF, Morton A, et al. CD4+ memory T cells are the predominant population of HIV-1-infected lymphocytes in neonates and children. AIDS. 1996;10:1477–84.
Alexander M, Lynch R, Mulenga J, et al. Donor and recipient envs from heterosexual human immunodeficiency virus subtype C transmission pairs require high receptor levels for entry. J Virol. 2010;84:4100–4.
Isaacman-Beck J, Hermann EA, Yi Y, et al. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol. 2009;83:8208–20.
Gartner S, Markovits P, Markovitz DM, et al. The role of mononuclear phagocytes in HTLV-III LAV infection. Science. 1986;233:215–9.
Brown RJP, Peters PJ, Caron C, et al. Intercompartmental recombination of HIV-1 contributes to env intrahost diversity and modulates viral tropism and sensitivity to entry inhibitors. J Virol. 2011;85:6024–37.
Peters PJ, Sullivan WM, Duenas-Decamp MJ, et al. Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J Virol. 2006;80:6324–32.
Thomas ER, Dunfee RL, Stanton J, et al. Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology. 2007;360:105–19.
• Schnell G, Joseph S, Spudich SS, et al. Two classes of viral encephalitis contribute to the development of HIV-1-associated dementia. PLoS Pathog In press. This paper demonstrates the production of macrophage-tropic virus from a long-lived cell in a subset of subjects with HIV-associated dementia.
• Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552-7. This paper demonstrates that in most transmissions of HIV-1, the systemic infection is derived from a single viral genome.
Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–67.
Fischer W, Ganusov VV, Giorgi EE, et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One. 2010;5:e12303.
Li H, Bar KJ, Wang S, et al. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 2010;6:e1000890.
Gottlieb GS, Nickle DC, Jensen MA, et al. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363:619–22.
Anderson JA, Ping LH, Dibben O, et al. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog. 2010;6:e1001053.
Chohan B, Lang D, Sagar M, et al. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol. 2005;79:6528–31.
Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–22.
Russell ES, Kwiek JJ, Keys J, et al. The genetic bottleneck in vertical transmission of subtype C HIV-1 Is not driven by selection of especially neutralization resistant virus from the maternal viral population. J Virol 2011;8253-62.
Nawaz F, Cicala C, Van Ryk D, et al. The genotype of early-transmitting HIV gp120s promotes alphabeta-reactivity, revealing alphabetaCD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7:e1001301.
Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–9.
Moore PL, Gray ES, Morris L. Specificity of the autologous neutralizing antibody response. Curr Opin HIV AIDS. 2009;4:358–63.
Ince WL, Zhang L, Jiang Q, et al. Evolution of the HIV-1 env gene in the Rag2-/- gammaC-/- humanized mouse model. J Virol. 2010;84:2740–52.
Pugach P, Kuhmann SE, Taylor J, et al. The prolonged culture of human immunodeficiency virus type 1 in primary lymphocytes increases its sensitivity to neutralization by soluble CD4. Virology. 2004;321:8–22.
Shibata J, Yoshimura K, Honda A, et al. Impact of V2 mutations on escape from a potent neutralizing anti-V3 monoclonal antibody during in vitro selection of a primary human immunodeficiency virus type 1 isolate. J Virol. 2007;81:3757–68.
Binley JM, Ban YE, Crooks ET, et al. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol. 2010;84:5637–55.
Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12.
Bosch ML, Andeweg AC, Schipper R, Kenter M. Insertion of N-linked glycosylation sites in the variable regions of the human immunodeficiency virus type 1 surface glycoprotein through AAT triplet reiteration. J Virol. 1994;68:7566–9.
Kitrinos KM, Hoffman NG, Nelson JA, Swanstrom R. Turnover of env variable region 1 and 2 genotypes in subjects with late-stage human immunodeficiency virus type 1 infection. J Virol. 2003;77:6811–22.
Koot M, Keet IP, Vos AH, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–8.
Connor RI, Sheridan KE, Ceradini D, et al. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–8.
Hunt PW, Harrigan PR, Huang W, et al. Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremia. J Infect Dis. 2006;194:926–30.
Bleul CC, Wu L, Hoxie JA, et al. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–30.
Lee B, Sharron M, Montaner LJ, et al. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96:5215–20.
Blaak H, van’t Wout AB, Brouwer M, et al. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc Natl Acad Sci U S A. 2000;97:1269–74.
Heeregrave EJ, Geels MJ, Brenchley JM, et al. Lack of in vivo compartmentalization among HIV-1 infected naive and memory CD4+ T cell subsets. Virology. 2009;393:24–32.
van Rij RP, Blaak H, Visser JA, et al. Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. J Clin Invest. 2000;106:1569.
Ince WL, Harrington PR, Schnell GL, et al. Major coexisting human immunodeficiency virus type 1 env gene subpopulations in the peripheral blood are produced by cells with similar turnover rates and show little evidence of genetic compartmentalization. J Virol. 2009;83:4068–80.
Hwang SS, Boyle TJ, Lyerly HK, Cullen BR. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–4.
de Jong JJ, Goudsmit J, Keulen W, et al. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992;66:757–65.
Milich L, Margolin BH, Swanstrom R. Patterns of amino acid variability in NSI-like and SI-like V3 sequences and a linked change in the CD4-binding domain of the HIV-1 Env protein. Virology. 1997;239:108–18.
Jensen MA, Li FS, van ’t Wout AB, et al. Improved coreceptor usage prediction and genotypic monitoring of R5-to-X4 transition by motif analysis of human immunodeficiency virus type 1 env V3 loop sequences. J Virol. 2003;77:13376–88.
Resch W, Hoffman N, Swanstrom R. Improved success of phenotype prediction of the human immunodeficiency virus type 1 from envelope variable loop 3 sequence using neural networks. Virology. 2001;288:51–62.
Hoffman NG, Seillier-Moiseiwitsch F, Ahn J, et al. Variability in the human immunodeficiency virus type 1 gp120 Env protein linked to phenotype-associated changes in the V3 loop. J Virol. 2002;76:3852–64.
Huang W, Toma J, Fransen S, et al. Coreceptor tropism can be influenced by amino acid substitutions in the gp41 transmembrane subunit of human immunodeficiency virus type 1 envelope protein. J Virol. 2008;82:5584–93.
Tscherning C, Alaeus A, Fredriksson R, et al. Differences in chemokine coreceptor usage between genetic subtypes of HIV-1. Virology. 1998;241:181–8.
Huang W, Eshleman SH, Toma J, et al. Coreceptor tropism in human immunodeficiency virus type 1 subtype D: high prevalence of CXCR4 tropism and heterogeneous composition of viral populations. J Virol. 2007;81:7885–93.
Sina ST, Ren W, Cheng-Mayer C. Coreceptor use in nonhuman primate models of HIV infection. J Transl Med. 2011;9 Suppl 1:S7.
Ho SH, Tasca S, Shek L, et al. Coreceptor switch in R5-tropic simian/human immunodeficiency virus-infected macaques. J Virol. 2007;81:8621–33.
Nishimura Y, Shingai M, Willey R, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–81.
Ren W, Tasca S, Zhuang K, et al. Different tempo and anatomic location of dual-tropic and X4 virus emergence in a model of R5 simian-human immunodeficiency virus infection. J Virol. 2010;84:340–51.
Ho SH, Shek L, Gettie A, et al. V3 loop-determined coreceptor preference dictates the dynamics of CD4 + -T-cell loss in simian-human immunodeficiency virus-infected macaques. J Virol. 2005;79:12296–303.
Nishimura Y, Igarashi T, Donau OK, et al. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc Natl Acad Sci U S A. 2004;101:12324–9.
Dunfee RL, Thomas ER, Gorry PR, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A. 2006;103:15160–5.
Dunfee RL, Thomas ER, Wang J, et al. Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology. 2007;367:222–34.
Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol. 2005;79:7959–66.
Harrington PR, Schnell G, Letendre SL, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23:907–15.
Pillai SK, Pond SLK, Liu Y, et al. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain. 2006;129:1872–83.
Ritola K, Robertson K, Fiscus SA, et al. Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol. 2005;79:10830–4.
• Xu Y, Zhu H, Wilcox CK, et al. Blood monocytes harbor HIV type 1 strains with diversified phenotypes including macrophage-specific CCR5 virus. J Infect Dis 2008;197:309–18. This is a provocative report identifying virus in monocytes that have lost the ability to replicate in T cells.
Li S, Juarez J, Alali M, et al. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73:9741–55.
Sterjovski J, Roche M, Churchill MJ, et al. An altered and more efficient mechanism of CCR5 engagement contributes to macrophage tropism of CCR5-using HIV-1 envelopes. Virology. 2010;404:269–78.
Taylor BM, Foulke JS, Flinko R, et al. An alteration of human immunodeficiency virus gp41 leads to reduced CCR5 dependence and CD4 independence. J Virol. 2008;82:5460–71.
Peters PJ, Duenas-Decamp MJ, Sullivan WM, et al. Variation in HIV-I R5 macrophage-tropism correlates with sensitivity to reagents that block envelope: CD4 interactions but not with sensitivity to other entry inhibitors. Retrovirology. 2008;5:5.
Roche M, Jakobsen MR, Sterjovski J, et al. HIV-1 escape from the CCR5 antagonist maraviroc associated with an altered and less-efficient mechanism of gp120-CCR5 engagement that attenuates macrophage tropism. J Virol. 2011;85:4330–42.
Sterjovski J, Churchill MJ, Ellett A, et al. Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS. Retrovirology. 2007;4:89.
Duenas-Decamp MJ, Peters P, Burton D, Clapham PR. Natural resistance of human immunodeficiency virus type 1 to the CD4bs antibody b12 conferred by a glycan and an arginine residue close to the CD4 binding loop. J Virol. 2008;82:5807–14.
Dunfee RL, Thomas ER, Gabuzda D. Enhanced macrophage tropism of HIV in brain and lymphoid tissues is associated with sensitivity to the broadly neutralizing CD4 binding site antibody b12. Retrovirology. 2009;6:69.
Duenas-Decamp MJ, Peters PJ, Burton D, Clapham PR. Determinants flanking the CD4 binding loop modulate macrophage tropism of human immunodeficiency virus type 1 R5 envelopes. J Virol. 2009;83:2575–83.
• Zhuang K, Finzi A, Tasca S, et al. Adoption of an “Open” envelope conformation facilitating CD4 binding and structural remodeling precedes coreceptor switch in R5 SHIV-infected macaques. Plos One 2011;6:e21350. This paper reports a new cell line where CD4 and CCR5 are under control of regulatable promoters, allowing quantitative assessment of the need for these receptors in entry.
Johnston SH, Lobritz MA, Nguyen S, et al. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J Virol. 2009;83:11016–26.
Duenas-Decamp MJ, Clapham PR. HIV-1 gp120 determinants proximal to the CD4 binding site shift protective glycans that are targeted by monoclonal antibody 2G12. J Virol. 2010;84:9608–12.
Richards KH, Aasa-Chapman MMI, McKnight A, Clapham PR. Modulation of HIV-1 macrophage-tropism among R5 envelopes occurs before detection of neutralizing antibodies. Retrovirology. 2010;7:48.
Musich T, Peters PJ, Duenas-Decamp MJ, et al. A conserved determinant in the V1 Loop of HIV-1 modulates the V3 loop to prime low CD4 use and macrophage infection. J Virol. 2011;85:2397–405.
Walter BL, Wehrly K, Swanstrom R, et al. Role of low CD4 levels in the influence of human immunodeficiency virus type 1 envelope V2 and V2 regions on entry and spread in macrophages. J Virol. 2005;79:4828–37.
Cashin K, Roche M, Sterjovski J, et al. Alternative coreceptor requirements for efficient CCR5- and CXCR4-mediated HIV-1 entry into macrophages. J Virol In press.
Ghaffari G, Tuttle DL, Briggs D, et al. Complex determinants in human immunodeficiency virus type 1 envelope gp120 mediate CXCR4-dependent infection of macrophages. J Virol. 2005;79:13250–61.
Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92.
Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leuk Biol. 2001;69:11–20.
Ellery PJ, Tippett E, Chiu YL, et al. The CD16(+) monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–9.
Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31.
Crowe S, Zhu TF, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leuk Biol. 2003;74:635–41.
Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–7.
Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:E74–80.
Naif HM, Li S, Alali M, et al. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–6.
Sonza S, Maerz A, Uren S, et al. Susceptibility of human monocytes to HIV type 1 infection in vitro is not dependent on their level of CD4 expression. AIDS Res Hum Retroviruses. 1995;11:769–76.
Dong C, Kwas C, Wu L. Transcriptional restriction of human immunodeficiency virus type 1 gene expression in undifferentiated primary monocytes. J Virol. 2009;83:3518–27.
Sung T-L, Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. Plos Pathog. 2009;5:e1000263.
Lewin SR, Lambert P, Deacon NJ, et al. Constitutive expression of p50 homodimer in freshly isolated human monocytes decreases in vitro and in vivo differentiation: a possible mechanism influencing human immunodeficiency virus replication in monocytes and mature macrophages. J Virol. 1997;71:2114–9.
Arfi V, Riviere L, Jarrosson-Wuilleme L, et al. Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1. J Virol. 2008;82:6557–65.
Meuret G, Bammert J, Hoffmann G. Kinetics of human monocytopoiesis. Blood. 1974;44:801–16.
Zhu TF, Muthui D, Holte S, et al. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–16.
Sonza S, Mutimer HP, Oelrichs R, et al. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS. 2001;15:17–22.
Riddick NE, Hermann EA, Loftin LM, et al. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010;6:e1001064.
Dumonceaux J, Nisole S, Chanel C, et al. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–9.
Haim H, Strack B, Kassa A, et al. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. Plos Pathog. 2011;7:e1002101.
Hoffman TL, LaBranche CC, Zhang WT, et al. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci U S A. 1999;96:6359–64.
Kolchinsky P, Mirzabekov T, Farzan M, et al. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–6.
Marras D, Bruggeman LA, Gao F, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nature Medicine. 2002;8:522–6.
Edwards TG, Hoffman TL, Baribaud F, et al. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J Virol. 2001;75:5230–9.
Eitner F, Cui Y, Hudkins KL, et al. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J Am Soc Nephrol. 2000;11:856–67.
Bruggeman LA, Ross MD, Tanji N, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–87.
Acknowledgments
We would like to thank the NIH for funding that supports our ongoing research in this field. We also apologize to our colleagues whose work we did not cite due to editorial limitations.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arrildt, K.T., Joseph, S.B. & Swanstrom, R. The HIV-1 Env Protein: A Coat of Many Colors. Curr HIV/AIDS Rep 9, 52–63 (2012). https://doi.org/10.1007/s11904-011-0107-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-011-0107-3