Abstract
Purpose of review
This review aims to highlight recent research on the gut microbiome in IBD and the application of microbiome-modulating therapies for the treatment of IBD including the use of the microbiome as an indicator for disease severity and treatment response.
Recent findings
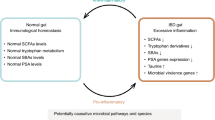
Despite the high number of gut microbiome studies and emerging evidence supporting the gut microbiome’s involvement in disease pathogenesis, no single microorganism has been identified as a pathogenic agent in IBD. Retrospective studies and meta-analyses on antibiotic use in ulcerative colitis and Crohn’s disease and long-term outcomes are conflicting. Similarly, the use of probiotics for the treatment of IBD remains inconclusive; however, some encouraging results are emerging as microbial concoctions are optimized to include beneficial bacterial strains. Fecal microbial transplantation (FMT) is currently emerging as one of the more promising microbiome-modulating IBD therapies. FMT studies in ulcerative colitis have shown improved remission rates compared to placebo; however, relatively small study sample sizes and varied treatment methods, limit definitive conclusions.
Summary
With clear evidence of an IBD gut dysbiosis, novel therapies to treat and prevent disease relapse will undoubtedly require a microbiome-modulating approach. The complexity and variability of IBD disease pathogenesis (disease phenotype, gut microbiome, host genetic susceptibility, and environmental factors) will likely require a personalized and multidimensional treatment approach where microbiome-modulating therapy is coupled with other therapies to target other IBD disease components.
Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- CDI:
-
Clostridium difficile infection
- EEN:
-
exclusive enteral nutrition (EEN)
- IBD:
-
inflammatory bowel disease
- FMT:
-
fecal microbiota transplantation
- MD-index:
-
microbial dysbiosis index
- SCD:
-
specific carbohydrate diet
- UC:
-
ulcerative colitis
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78. This review updates current epidemiological data on IBD incidence and prevalence from around the world, highlighting the ongoing emergence of IBD in the developing world.
de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27.
Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naïve microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–92.
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11.
Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15:653–60.
Moustafa A, Li W, Anderson EL, Wong EHM, Dulai PS, Sandborn WJ, et al. Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clin Transl Gastroenterol. 2018;9:e132.
Liguori G, Lamas B, Richard ML, Brandi G, da Costa G, Hoffmann TW, et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J Crohns Col. 2016;10:296–305.
Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, et al. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion. 2016;93:59–65.
Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854.e1.
Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–18.
Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut. 2007;56:669–75.
Fang X, Monk J, Nurk S, Akseshina M, Zhu Q, Gemmell C, et al. Metagenomics-based, strain-level analysis of Escherichia coli from a time-series of microbiome samples from a Crohn’s disease patient. Front Microbiol [Internet]. 2018 [cited 2018 Oct 18];9. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02559/abstract. Accessed 1 Nov 2018.
Tahara T, Shibata T, Kawamura T, Okubo M, Ichikawa Y, Sumi K, et al. Fusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in Japan. Dig Dis Sci. 2015;60:205–10.
Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79–83.
Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, DeVinney R, et al. Invasive potential of gut mucosa-derived fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17:1971–8.
• Imhann F, Vila AV, Bonder MJ, Fu J, Gevers D, Visschedijk MC, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–19. This study investigated the link between the IBD host genotype and gut microbiome using 16S rRNA sequencing of stool samples and host genotyping data in comparison to healthy controls. A key finding from this study was evidence that a gut dysbiosis commonly associated with IBD may precede disease manifestation. Healthy controls with high IBD genetic susceptibility were significantly associated with a lower abundance of Roseburia.
•• Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–5. Using independent cohorts, this study provides evidence that the influence of host genetics on the gut microbiome is likely minimal and report that only a small proportion of the gut microbiome is heritable; environmental factors impart a much larger effect on the human gut microbiome composition.
•• Schirmer M, Franzosa EA, Lloyd-Price J, LJ MI, Schwager R, Poon TW, et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nature Microbiol. 2018;3:337–46. One of the first studies to combine both metagenomics and metatranscriptomics approaches on fecal samples taken from IBD patients and healthy controls. Their results highlight variability in the sequence data generated from both approaches suggesting that the transcriptional activity may unravel additional disease mechanisms not possible through microbial DNA sequence abundance data.
Rehman A, Lepage P, Nolte A, Hellmig S, Schreiber S, Ott SJ. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J Med Microbiol. 2010;59:1114–22.
Sokol H, Leducq V, Aschard H, Pham H-P, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–48.
Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, et al. A fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1948–56.
Mukhopadhya I, Hansen R, Meharg C, Thomson JM, Russell RK, Berry SH, et al. The fungal microbiota of de-novo paediatric inflammatory bowel disease. Microbes Infect. 2015;17:304–10.
El Mouzan M, Wang F, Al Mofarreh M, Menon R, Al Barrag A, Korolev KS, et al. Fungal microbiota profile in newly diagnosed treatment-naïve children with Crohn’s disease. J Crohn Col. 2017;11:586–92.
Seider K, Gerwien F, Kasper L, Allert S, Brunke S, Jablonowski N, et al. Immune evasion, stress resistance, and efficient nutrient acquisition are crucial for intracellular survival of Candida glabrata within macrophages. Eukaryot Cell. 2014;13:170–83.
Dollive S, Chen Y-Y, Grunberg S, Bittinger K, Hoffmann C, Vandivier L, et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS One. 2013;8:e71806.
Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–60.
Ungaro F, Massimino L, Furfaro F, Rimoldi V, Peyrin-Biroulet L, D’Alessio S, et al. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes. 2018;0:1–10.
Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and Meta-analysis. Am J Gastroenterol. 2011;106:661–73.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. PNAS. 2011;108:4554–61.
•• Levine A, Kori M, Kierkus J, Boneh RS, Sladek M, Escher JC, et al. Azithromycin and metronidazole versus metronidazole-based therapy for the induction of remission in mild to moderate paediatric Crohn’s disease : a randomised controlled trial. Gut. 2018;68:gutjnl-2017–315199. This study was a randomized controlled trial of azithromycin plus metronidazole versus metronidazole alone in children with Crohn’s disease. The group randomized to azithromycin had a higher remission rate. The remission rate in the azithromycin group was sufficiently high to raise concerns about the impact of lack of blinding.
Gupta V, Rodrigues R, Nguyen D, Sauk J, Khalili H, Yajnik V, et al. Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Therap. 2016;43:52–60.
Ledder O, Turner D. Antibiotics in IBD: still a role in the biological era? Inflamm Bowel Dis. 2018;24:1676–88.
Bernstein CN. Antibiotics, Probiotics and Prebiotics in IBD. Nutrition, gut microbiota and immunity: therapeutic targets for IBD. Nestle Nutr Inst Workshop Ser. 2014;79:83–100.
Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Therap. 2017;46:389–400.
Sivignon A, de Vallée A, Barnich N, Denizot J, Darcha C, Pignède G, et al. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn’s disease. Inflamm Bowel Dis. 2015;21:276–86.
•• Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–28. This was the most recent published randomized placebo controlled trial of fecal microbial transplantation in the treatment of UC. It suggested a magnitude of benefit similar to that seen in the previously reported Canadian study.
Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–9.
Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JHA, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.e4.
•• Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–93. This randomized controlled trial showed that encapsulated stool was comparably effective at reducing recurrence of C difficile as colonoscopically administered fecal microbial transplantation.
Narula N, Kassam Z, Yuan Y, Colombel J-F, Ponsioen C, Reinisch W, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017;23:1702–9.
Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohn Col. 2017;11:1180–99.
Qazi T, Amaratunga T, Barnes EL, Fischer M, Kassam Z, Allegretti JR. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: systematic review and meta-analysis. Gut Microbes. 2017;8:574–88.
Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:410–21.
Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–8.
Debelius J, Song SJ, Vazquez-Baeza Y, Xu ZZ, Gonzalez A, Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies? Genome Biol [Internet]. 2016 [cited 2018 Oct 28];17. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5072314/. Accessed 1 Nov 2018.
Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, et al. A microbial signature for Crohn’s disease. Gut. 2017;66:813–22.
Gevers D, Kugathasan S, Knights D, Kostic AD, Knight R, Xavier RJ. A microbiome foundation for the study of Crohn’s disease. Cell Host Microbe. 2017;21:301–4.
Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63–73.
Suskind DL, Cohen SA, Brittnacher MJ, Wahbeh G, Lee D, Shaffer ML, et al. Clinical and fecal microbial changes with diet therapy in active inflammatory bowel disease. J Clin Gastroenterol. 2018;52:155–63.
Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861–71.
Ashton JJ, Colquhoun CM, Cleary DW, Coelho T, Haggarty R, Mulder I, et al. 16S sequencing and functional analysis of the fecal microbiome during treatment of newly diagnosed pediatric inflammatory bowel disease. Medicine (Baltimore) [Internet]. 2017 [cited 2018 Oct 3];96. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500076/Accessed 1 Nov 2018.
Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in Inflammatory bowel diseases. Cell Host Microbe. 2017;21:603–610.e3.
Funding
Gary Van Domselaar receives operational funding from the Public Health Agency of Canada and has also received research support from the Multiple Sclerosis Society of Canada, the Canadian Institutes of Health Research, The National Sciences and Engineering Research Council, and Genome Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Charles Bernstein is supported in part by the Bingham Chair in Gastroenterology. He has served on advisory boards of Abbvie Canada, Ferring Canada, Janssen Canada, Pfizer Canada, Shire Canada, Takeda Canada, Napo Pharmaceuticals, and has consulted to 4D Pharm and Mylan Pharmaceuticals. He has received educational grants from Abbvie Canada, Janssen Canada, Pfizer Canada, Shire Canada, and Takeda Canada.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Editor Gary Lichtenstein
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Inflammatory Bowel Disease
Rights and permissions
About this article
Cite this article
Knox, N.C., Forbes, J.D., Van Domselaar, G. et al. The Gut Microbiome as a Target for IBD Treatment: Are We There Yet?. Curr Treat Options Gastro 17, 115–126 (2019). https://doi.org/10.1007/s11938-019-00221-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00221-w