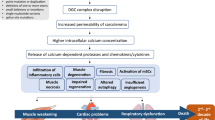
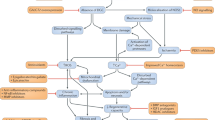
Abstract
Purpose of review
The purpose of this review is to summarize the current and emerging therapies for Duchenne muscular dystrophy (DMD).
Recent findings
Coinciding with new standardized care guidelines, there are a growing number of therapeutic options to treat males with DMD. Treatment of the underlying pathobiology, such as micro-dystrophin gene replacement, exon skipping, stop codon read-through agents, and utrophin modulators showed variable success in animal and human studies. Symptomatic therapies to target muscle ischemia, enhance muscle regeneration, prevent muscle fibrosis, inhibit myostatin, and reduce inflammation are also under investigation.
Summary
DMD is a complex, heterogeneous degenerative disease. The pharmacological and technological achievements made in recent years, plus timely supportive interventions will likely lead to an improved quality of life for many individuals with DMD.
Similar content being viewed by others
Abbreviations
- 6MWT:
-
6-Min walk test
- AAV:
-
Adeno-associated virus
- ACE:
-
Angiotensin-converting enzyme
- AON:
-
Antisense oligonucleotide
- BMD:
-
Becker muscular dystrophy
- CGH:
-
Comparative genomic hybridization microarray
- CTGF:
-
Connective tissue growth factor
- CK:
-
Creatine kinase
- DEXA:
-
Dual-energy X-ray absorptiometry
- DMD:
-
Duchenne muscular dystrophy
- DNA:
-
Deoxyribonucleic acid
- ECHO:
-
Echocardiogram
- ECG:
-
Electrocardiogram
- FDA:
-
Food and Drug Administration
- FVC:
-
Forced vital capacity
- GCSF:
-
Granulocyte colony-stimulating factor
- MLPA:
-
Multiplex ligation-dependent probe amplification
- MRI:
-
Magnetic resonance imaging
- NCT:
-
National clinical trial
- NIPPV:
-
Non-invasive intermittent positive pressure ventilation
- NF-κB:
-
Nuclear factor-kappa B
- OSA:
-
Obstructive sleep apnea
- %PPEF:
-
Percentage predicted peak expiratory flow
- RNA:
-
Ribonucleic acid
- SDB:
-
Sleep disordered breathing
- TGF-β:
-
Transforming growth factor beta
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28.
Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord. 1991;1(1):19–29.
• Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders. 2014;24(6):482–91. https://doi.org/10.1016/j.nmd.2014.03.008. This was the first review of world-wide incidence and prevalence of Duchene muscular dystrophy.
Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53(2):219–28.
Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, Kosma K, et al. The TREAT-NMD DMD global database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat. 2015;36(4):395–402. https://doi.org/10.1002/humu.22758.
Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, Van Ommen G-JB, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135–44. https://doi.org/10.1002/mus.20586.
Mah JK, Selby K, Campbell C, Nadeau A, Tarnopolsky M, McCormick A, et al. A population-based study of dystrophin mutations in Canada. Canadian Journal of Neurological Sciences / Journal Canadien Des Sciences Neurologiques. 2011;38(3):465–74. https://doi.org/10.1017/S0317167100011896.
Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989;45(4):498–506.
Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–5.
Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci. 2011;108(2):762–7. https://doi.org/10.1073/pnas.1013067108.
Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2007;1772(2):108–17. https://doi.org/10.1016/j.bbadis.2006.05.010.
Gao QQ, McNally EM. The dystrophin complex: structure, function, and implications for therapy. In: Terjung R, editor. Comprehensive physiology. Hoboken: John Wiley & Sons, Inc.; 2015. p. 1223–39.
Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2(12):731–40.
Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88(2):83–91. https://doi.org/10.1139/Y09-058.
Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71(1):37–57. https://doi.org/10.1146/annurev.physiol.010908.163216.
Thomas GD. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front Physiol. 2013;4:1–6.
•• Bushby K, Finkel R, Birnkrant D, et al. Diagnosis and management of Duchenne muscular dystrophy part 1: diagnosis and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. These expert-led international guidelines provided recommendations for the diagnosis and medical management of Duchenne muscular dystrophy.
McDonald CM, Henricson EK, Abresch RT, Han JJ, Escolar DM, Florence JM. The Cinrg investigators. The cooperative international neuromuscular research group Duchenne natural history study-a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used: CINRG DMD study. Muscle Nerve. 2013;48(1):32–54. https://doi.org/10.1002/mus.23807.
Darras B, Menache-Starobinski C, Hinton V, Kunkel L. Dystrinopathies. Chapter 30. In: Darras B, Jones H, Ryan M, De Vivo D, eds. Neuromuscular disorders of infancy, childhood, and adolescence a clinicians approach, 2nd edn. London: Elsevier, 2015; 551–92.
Pane M, Lombardo ME, Alfieri P, D’Amico A, Bianco F, Vasco G, et al. Attention deficit hyperactivity disorder and cognitive function in Duchenne muscular dystrophy: phenotype-genotype correlation. J Pediatr. 2012;161(4):705–709.e1. https://doi.org/10.1016/j.jpeds.2012.03.020.
Leibowitz D, Dubowitz V. Intellect and behavior in Duchenne muscular dystrophy. Develop Med Child Neurol. 1981;23:577–90.
Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–9.
Judge DP, Kass DA, Thompson WR, Wagner KR. Pathophysiology and therapy of cardiac dysfunction in Duchenne muscular dystrophy. Am J Cardiovasc Drugs. 2011;11(5):287–94.
Nigro G, Comi LI, Politano L, Bain RJI. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26(3):271–7.
•• Bushby K, Finkel R, Birnkrant D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9(2):177–89. These expert-led international guidelines provided recommendations for the multidisciplinary management of Duchenne muscular dystrophy.
•• Birnkrant D, Bushby K, Bann C, Alman B, Apkon S, Blackwell A, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347–61. This expert-led guideline, updated from 2010, reflects the current literature with particular attention to respiratory, endocrinological, and rehabilitation care.
Suresh S, Wales P, Dakin C, Harris M-A, Cooper DGM. Sleep-related breathing disorder in Duchenne muscular dystrophy: disease spectrum in the paediatric population. J Paediatr Child Health. 2005;41(9–10):500–3.
Ragette R, Mellies U, Schwake C, Voit T, Teschler H. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax. 2002;57(8):724–8.
Yiu EM, Kornberg AJ. Duchenne muscular dystrophy: Duchenne muscular dystrophy. J Paediatr Child Health. 2015;51(8):759–64. https://doi.org/10.1111/jpc.12868.
Rodillo EB, Fernandez-Bermejo E, Heckmatt JZ, Dubowitz V. Prevention of rapidly progressive scoliosis in Duchenne muscular dystrophy by prolongation of walking with orthoses. J Child Neurol. 1988;3(4):269–74.
Nallamilli B, Ankala A, Hegde M. Molecular diagnosis of Duchenne muscular dystrophy. Curr Protoc Hum Genet. 2014;83:9251–92529.
Hegde MR, Chin ELH, Mulle JG, Okou DT, Warren ST, Zwick ME. Microarray-based mutation detection in the dystrophin gene. Hum Mutat. 2008;29(9):1091–9. https://doi.org/10.1002/humu.20831.
Center for Disease Control. Survival of males diagnosed with Duchenne/Becker muscular dystrophy (DBMD) by years of birth—muscular dystrophy surveillance tracking and research network. MMWR. 2009;58(40):1119–22.
Aartsma-Rus A, Ginjaar IB, Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53(3):145–51. https://doi.org/10.1136/jmedgenet-2015-103387.
Villanova M, Brancalion B, Mehta AD. Duchenne muscular dystrophy: life prolongation by noninvasive ventilatory support. Am J Phys Med Rehabil. 2014;93(7):595–9. https://doi.org/10.1097/PHM.0000000000000074.
• Matthews, E., Brassington, R., Kuntzer, T., Jichi, F., & Manzur, A. Y. (2016). Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews. doi: https://doi.org/10.1002/14651858.CD003725.pub4. A Cochrane review that evaluated all glucocorticoid studies in DMD.
Moxley RT, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64(1):13–20.
Balaban B, Matthews DJ, Clayton GH, Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84(11):843–50. https://doi.org/10.1097/01.phm.0000184156.98671.d0.
Gloss D, Moxley RT, Ashwal S, Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2016;86(5):465–72.
Angelini C, Peterle E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myologica. 2012;31(1):9–15.
Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing: CINRG DMD natural history study. Muscle Nerve. 2013;48(1):55–67. https://doi.org/10.1002/mus.23808.
Pane M, Fanelli L, Mazzone ES, Olivieri G, D’Amico A, Messina S, et al. Benefits of glucocorticoids in non-ambulant boys/men with Duchenne muscular dystrophy: a multicentric longitudinal study using the performance of upper limb test. Neuromuscul Disord. 2015;25(10):749–53. https://doi.org/10.1016/j.nmd.2015.07.009.
Schara U, Mortier J, Mortier W. Long-term steroid therapy in Duchenne muscular dystrophy positive results versus side effects. J Clin Neuromuscul Dis. 2001;2(4):179–83.
McAdam LC, Mayo AL, Alman BA, Biggar WD. The Canadian experience with long term deflazacort treatment in Duchenne muscular dystrophy. Acta Myologica. 2012;31(1):16–20.
Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006;16(4):249–55. https://doi.org/10.1016/j.nmd.2006.01.010.
Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14(2):153–61.
Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, et al. Managing Duchenne muscular dystrophy—the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17(6):470–5. https://doi.org/10.1016/j.nmd.2007.03.002.
Passamano L, Taglia A, Palladino A, Viggiano E, D’AMBROSIO P, Scutifero M, et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myologica. 2012;31(2):121–5.
Hoogerwaard E, van der Wouw P, Wilde A, Bakker P, Ippel P, Oosterwijk J, et al. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1999;9:347–51.
Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care. 2011;56(6):744–50. https://doi.org/10.4187/respcare.00831.
Toussaint M, Steens M, Wasteels G, Soudon P. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J. 2006;28(3):549–55. https://doi.org/10.1183/09031936.06.00004906.
Birnkrant, D. J., Bushby, K. M. D., Amin, R. S., Bach, J. R., Benditt, J. O., Eagle, M., … Kravitz, R. M. The respiratory management of patients with Duchenne muscular dystrophy: a DMD care considerations working group specialty article. Pediatr Pulmonol, 2010;45(8), 739–748. doi: https://doi.org/10.1002/ppul.21254
Mendell J, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363(15):1429–37.
Shin J-H, Pan X, Hakim CH, Yang HT, Yue Y, Zhang K, et al. Microdystrophin ameliorates muscular dystrophy in the canine model of Duchenne muscular dystrophy. Mol Ther. 2013;21(4):750–7. https://doi.org/10.1038/mt.2012.283.
Okada T, Takeda S. Current challenges and future directions in recombinant AAV-mediated gene therapy of Duchenne muscular dystrophy. Pharmaceuticals. 2013;6(12):813–36. https://doi.org/10.3390/ph6070813.
Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat Rev Genet. 2013;14(6):373–8.
Guiraud S, Chen H, Burns DT, Davies KE. Advances in genetic therapeutic strategies for Duchenne muscular dystrophy: therapeutic strategies for Duchenne muscular dystrophy. Exp Physiol. 2015;100(12):1458–67. https://doi.org/10.1113/EP085308.
Yin H, Moulton HM, Betts C, Merritt T, Seow Y, Ashraf S, et al. Functional rescue of dystrophin-deficient mdx mice by a chimeric peptide-PMO. Mol Ther. 2010;18(10):1822–9. https://doi.org/10.1038/mt.2010.151.
Al-Zaidy S, Rodino-Klapac L, Mendell JR. Gene therapy for muscular dystrophy: moving the field forward. Pediatr Neurol. 2014;51(5):607–18. https://doi.org/10.1016/j.pediatrneurol.2014.08.002.
Malerba A, Boldrin L, Dickson G. Long-term systemic administration of unconjugated morpholino oligomers for therapeutic expression of dystrophin by exon skipping in skeletal muscle: implications for cardiac muscle integrity. Nucleic Acid Therapeutics (Formerly Oligonucleotides). 2011;21(4):293–8. https://doi.org/10.1089/nat.2011.0306.
Van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357(26):2677–86.
Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364(16):1513–22.
Goemans N, Mercuri E, Belousova E, Komaki H, Dubrovsky A, McDonald CM, et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28(1):4–15. https://doi.org/10.1016/j.nmd.2017.10.004.
Voit T, Topaloglu H, Straub V, Muntoni F, Deconinck N, Campion G, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory randomized placebo-controlled phase 2 study. Lancet Neurol. 2014;13(10):987–99.
Rowel K, Lim Q, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Design Development and Therapt. 2017;11:533–45.
Flanigan K, Voit T, Rosales X, Servais L, Kraus J, Wardell C, et al. Pharmacokinetics and safety of single doses of drisapersen in non-ambulant subjects with Duchenne muscular dystrophy: results of a double-blind randomized clinical trial. Neuromuscul Disord. 2014;24:16–24.
• Mendell J, Rodino-Klapac K, Sahenk Z. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74(5):637–47. This double-blinded placebo controlled study evaluated eteplirsen; it was the first FDA approved treatment for DMD.
Yu X, Bao B, Echigoya Y, Yokota T. Dystrophin-deficient large animal models: translation research and exon skipping. Am J Transl Res. 2015;7(8):1314–31.
Hodgkinson, L. (2016). Biosimilar Medicines Group-14th Annual Medicines for Europe Conference (April 28-29, 2016-London, UK). Drugs of today (Barcelona, Spain: 1998) 52, 309.
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–7.
Betts C, Saleh AF, Arzumanov AA, Hammond SM, Godfrey C, Coursindel T, et al. Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Molecular Therapy - Nucleic Acids. 2012;38:1–13. https://doi.org/10.1038/mtna.2012.30.
Goyenvalle A, Griffith G, Babbs A, Andaloussi SE, Ezzat K, Avril A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21(3):270–5. https://doi.org/10.1038/nm.3765.
Cirak S, Feng L, Anthony K, Arechavala-Gomeza V, Torelli S, Sewry C, et al. Restoration of the dystrophin-associated glycoprotein complex after exon skipping therapy in Duchenne muscular dystrophy. Mol Ther. 2012;20(2):462–7. https://doi.org/10.1038/mt.2011.248.
Wagner K, Hamed S, Hadley D, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsend mutations. Ann Neurol. 2001;49(6):706–11.
Politano L, Nigro G, Nigro V et al. Gentamicin administration in Duchenne patients with premature stop codon Preliminary results. Acta Myol 2003;(1):15–21.
Malik V, Rodino-Klapac LR, Viollet L, Mendell JR. Aminoglycoside-induced mutation suppression (stop codon readthrough) as a therapeutic strategy for Duchenne muscular dystrophy. Ther Adv Neurol Disord. 2010;3(6):379–89.
Malik V, Rodino-Klapac L, Viollet L, Wall C, King W, al-Dahhak R, et al. Gentamicin-induced read through of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010;67(6):771–80.
Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon- skipping in Duchenne dystrophy dogs. Ann Neurol. 2003;65:667–76.
Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605.
Finkel RS, Flanigan KM, Wong B, Bönnemann C, Sampson J, Sweeney HL, et al. Phase 2a study of Ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLoS One. 2013;8(12),e81302):1–10. https://doi.org/10.1371/journal.pone.0081302.
Bushby K, Finkel R, Wong B, Barohn R, Campbell C, Comi GP. FOR THE PTC124-GD-007-DMD STUDY GROUP.Ataluren treatment of patients with nonsense mutation dystrophinopathy: Ataluren for dystrophinopathy. Muscle Nerve. 2014;50(4):477–87. https://doi.org/10.1002/mus.24332.
Haas M, Vlcek V, Balabanov P, Salmonson T, Bakchine S, Markey G, et al. European Medicines Agency review of Ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord. 2015;25(1):5–13. https://doi.org/10.1016/j.nmd.2014.11.011.
Cox DBT, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–31. https://doi.org/10.1038/nm.3793.
Vanden Driessche T, Chuah MK. CRISPR/Cas9 flexes its muscles: in vivo somatic gene editing for muscular dystrophy. Mol Ther. 2016;24(3):414–6. https://doi.org/10.1038/mt.2016.29.
Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz EO, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351(6271):400–3. https://doi.org/10.1126/science.aad5725.
Mendell JR, Rodino-Klapac LR. Duchenne muscular dystrophy: CRISPR/Cas9 treatment. Cell Res. 2016;26(5):513–4.
Ousterout DG, Kabadi AM, Thakore PI, Majoros WH, Reddy TE, Gersbach CA. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun. 2015;6(1):1–32. https://doi.org/10.1038/ncomms7244.
Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment—relevance for therapy. FEBS J. 2013;280(17):4281–93.
Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, et al. Intra-arterial transplantation of hla-matched donor mesioangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015;7(12):1513–28.
Kleopa KA, Drousiotou A, Mavrikiou E, Ormiston A, Kyriakides T. Naturally occurring utrophin correlates with disease severity in Duchenne muscular dystrophy. Hum Mol Genet. 2006;15(10):1623–8. https://doi.org/10.1093/hmg/ddl083.
Guiraud S, Squire SE, Edwards B, Chen H, Burns DT, Shah N, et al. (2015). Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum Mol Genet. 2015;24(15):4212–24. https://doi.org/10.1093/hmg/ddv154.
Ricotti V, Spinty S, Roper H, Hughes I, Tejura B, Robinson N, et al. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-Arylbenzoxazole utrophin modulator, following single-and multiple-dose administration to pediatric patients with Duchenne muscular dystrophy. PLoS One. 2016;11(4):1–16.e0152840
Buyse GM, Goemans N, van den Hauwe M, Thijs D, de Groot IJM, Schara U, et al. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: results from a 12-month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord. 2011;21(6):396–405. https://doi.org/10.1016/j.nmd.2011.02.016.
Buyse GM, Goemans N, van den Hauwe M, Meier T. Effects of glucocorticoids and idebenone on respiratory function in patients with Duchenne muscular dystrophy. Pediatr Pulmonol. 2013;48(9):912–20. https://doi.org/10.1002/ppul.22688.
Buyse GM, Voit T, Schara U, Straathof CS, D’Angelo MG, Bernert G, et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet. 2015;385(9979):1748–57.
Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects: VBP15 improves muscular dystrophy. EMBO Molecular Medicine. 2013;5(10):1569–85. https://doi.org/10.1002/emmm.201302621.
Donovan J, Zimmer M, Offman E, Grant T, Jirousek M. A novel NF-KB inhibitor, Edasalonexent (CAT-1004) in development as a disease-modifying treatment for patients with Duchene muscular dystrophy: phase I safety, pharmacokinetics, and pharmacodynamics in adult subjects. Journal of Clin Pharm. 2017;57(5):627–39.
Whitehead NP, Pham C, Gervasio OL, Allen DG. N -acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice: reactive oxygen species and mdx muscle damage. J Physiol. 2008;586(7):2003–14. https://doi.org/10.1113/jphysiol.2007.148338.
Evans NP, Call JA, Bassaganya-Riera J, Robertson JL, Grange RW. Green tea extract decreases muscle pathology and NF-κB immunostaining in regenerating muscle fibers of mdx mice. Clin Nutr. 2010;29(3):391–8. https://doi.org/10.1016/j.clnu.2009.10.001.
Hibaoui Y, Reutenauer-Patte J, Patthey-Vuadens O, Ruegg UT, Dorchies OM. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy: melatonin improves dystrophic muscle function. J Pineal Res. 2011;51(2):163–71. https://doi.org/10.1111/j.1600-079X.2011.00871.
Escolar DM, Zimmerman A, Bertorini T, Clemens PR, Connolly AM, Mesa L, et al. Pentoxifylline as a rescue treatment for DMD a randomized double-blind clinical trial. Neurology. 2012;78(12):904–13.
Bettica P, Petrini S, D’Oria V, D’Amico A, Catteruccia M, Pane M, et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26(10):643–9. https://doi.org/10.1016/j.nmd.2016.07.002.
Consalvi S, Mozzetta C, Bettica P, Germani M, Fiorentini F, Del Bene F, et al. Preclinical studies in the mdx mouse model of Duchenne muscular dystrophy with the histone deacetylase inhibitor givinostat. Mol Med. 2003;19(1):79–87.
Hayashiji N, Yuasa S, Miyagoe-Suzuki Y, Hara M, Ito N, Hashimoto H, et al. G-CSF supports long-term muscle regeneration in mouse models of muscular dystrophy. Nat Commun. 2015;6(1):1–16. https://doi.org/10.1038/ncomms7745.
Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JAJ, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One. 2007;2(8):1–16 e806. https://doi.org/10.1371/journal.pone.0000806.
Hafner P, Bonati U, Erne B, Schmid M, Rubino D, Pohlman U, et al. Improved muscle function in Duchenne muscular dystrophy through l-arginine and metformin: an investigator-initiated, open-label, single-center, proof-of-concept-study. PLoS One. 2016;11(1):1–19.e0147634
Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62(10):1771–7.
Escolar DM, Buyse G, Henricson E, Leshner R, Florence J, Mayhew J, et al. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann Neurol. 2005;58(1):151–5. https://doi.org/10.1002/ana.20523.
Péladeau C, Ahmed A, Amirouche A, Crawford Parks TE, Bronicki LM, Ljubicic V, et al. Combinatorial therapeutic activation with heparin and AICAR stimulates additive effects on utrophin. An expression in dystrophic muscles. Hum Mol Genet. 2016;25(1):24–43. https://doi.org/10.1093/hmg/ddv444.
Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-β–induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13(2):204–10. https://doi.org/10.1038/nm1536.
Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, et al. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011;16(1):87–95. https://doi.org/10.1177/1074248410381757.
Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, et al. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22(24):4938–51. https://doi.org/10.1093/hmg/ddt352.
Cacchiarelli D, Martone J, Girardi E, Cesana M, Incitti T, Morlando M, et al. MicroRNAs involved in molecular circuitries relevant for the Duchenne muscular dystrophy pathogenesis are controlled by the dystrophin/nNOS pathway. Cell Metab. 2010;12(4):341–51. https://doi.org/10.1016/j.cmet.2010.07.008.
Bodanovsky A, Guttman N, Barzilai-Tutsch H, Genin O, Levy O, Pines M, et al. Halofuginone improves muscle-cell survival in muscular dystrophies. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2014;1843(7):1339–47. https://doi.org/10.1016/j.bbamcr.2014.03.025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Megan Crone received a research grant from the Alberta Children’s Hospital Research Institute.
Jean K. Mah received research support for DMD clinical trials from PTC Therapeutics, Pfizer, Bristol-Myers Squibb, RevereGen BioPharma, and NS Pharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuromuscular Disorders
Rights and permissions
About this article
Cite this article
Crone, M., Mah, J.K. Current and Emerging Therapies for Duchenne Muscular Dystrophy. Curr Treat Options Neurol 20, 31 (2018). https://doi.org/10.1007/s11940-018-0513-6
Published:
DOI: https://doi.org/10.1007/s11940-018-0513-6