Abstract
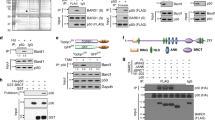
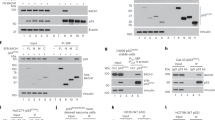
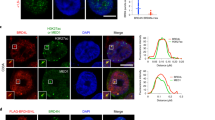
BRCA1-associated protein-1 (BAP1) is a 729 residue, nuclear-localized deubiquitinating enzyme (DUB) that displays tumor suppressor properties in the BAP1-null NCI-H226 lung carcinoma cell line. Studies that have altered BAP1 cellular levels or enzymatic activity have reported defects in cell cycle progression, notably at the G1/S transition. Recently BAP1 was shown to associate with the transcriptional regulator host cell factor 1 (HCF-1). The BAP1/HCF-1 interaction is mediated by the HCF-1 Kelch domain and an HCF-1 binding motif (HBM) within BAP1. HCF-1 is modified with ubiquitin in vivo, and ectopic studies suggest BAP1 deubiquitinates HCF-1. HCF-1 is a chromatin-associated protein thought to both activate and repress transcription by linking appropriate histone-modifying enzymes to a subset of transcription factors. One known role of HCF-1 is to promote cell cycle progression at the G1/S boundary by recruiting H3K4 histone methyltransferases to the E2F1 transcription factor so that genes required for S-phase can be transcribed. Given the robust associations between BAP1/HCF-1 and HCF-1/E2Fs, it is reasonable to speculate that BAP1 influences cell proliferation at G1/S by co-regulating transcription from HCF-1/E2F-governed promoters


Similar content being viewed by others
References
Ventii, K. H., Devi, N. S., Friedrich, K. L., Chernova, T. A., Tighiouart, M., Van Meir, E. G., et al. (2008). BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Research, 68, 6953–6962.
Jensen, D. E., Proctor, M., Marquis, S. T., Gardner, H. P., Ha, S. I., Chodosh, L. A., et al. (1998). BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene, 16, 1097–1112.
Nishikawa, H., Wu, W., Koike, A., Kojima, R., Gomi, H., Fukuda, M., et al. (2009). BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Research, 69, 111–119.
Machida, Y. J., Machida, Y., Vashisht, A. A., Wohlschlegel, J. A., & Dutta, A. (2009). The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. Journal of Biological Chemistry, 284, 34179–34188.
Sowa, M. E., Bennett, E. J., Gygi, S. P., & Harper, J. W. (2009). Defining the human deubiquitinating enzyme interaction landscape. Cell, 138, 389–403.
Misaghi, S., Ottosen, S., Izrael-Tomasevic, A., Arnott, D., Lamkanfi, M., Lee, J., et al. (2009). Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Molecular and Cellular Biology, 29, 2181–2192.
Tyagi, S., Chabes, A. L., Wysocka, J., & Herr, W. (2007). E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell, 27, 107–119.
Tyagi, S., & Herr, W. (2009). E2F1 mediates DNA damage and apoptosis through HCF-1 and the MLL family of histone methyltransferases. EMBO Journal, 28, 3185–3195.
Pickart, C. M. (2001). Mechanisms underlying ubiquitination. Annual Review of Biochemistry, 70, 503–533.
Hershko, A., & Ciechanover, A. (1998). The ubiquitin system. Annual Review of Biochemistry, 67, 425–479.
Hicke, L. (2001). Protein regulation by monoubiquitin. Nature Reviews Molecular Cell Biology, 2, 195–201.
Schwartz, A. L., & Ciechanover, A. (1999). The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annual Review of Medicine, 50, 57–74.
Komander, D. (2009). The emerging complexity of protein ubiquitination. Biochemical Society Transactions, 37, 937–953.
Kim, H. T., Kim, K. P., Lledias, F., Kisselev, A. F., Scaglione, K. M., Skowyra, D., et al. (2007). Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. Journal of Biological Chemistry, 282, 17375–17386.
Bernassola, F., Karin, M., Ciechanover, A., & Melino, G. (2008). The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell, 14, 10–21.
Reyes-Turcu, F. E., Ventii, K. H., & Wilkinson, K. D. (2009). Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annual Review of Biochemistry, 78, 363–397.
Nijman, S. M., Luna-Vargas, M. P., Velds, A., Brummelkamp, T. R., Dirac, A. M., Sixma, T. K., et al. (2005). A genomic and functional inventory of deubiquitinating enzymes. Cell, 123, 773–786.
Amerik, A. Y., & Hochstrasser, M. (2004). Mechanism and function of deubiquitinating enzymes. Biochimica et Biophysica Acta, 1695, 189–207.
Komander, D., Clague, M. J., & Urbe, S. (2009). Breaking the chains: structure and function of the deubiquitinases. Nature Reviews Molecular Cell Biology, 10, 550–563.
Larsen, C. N., Krantz, B. A., & Wilkinson, K. D. (1998). Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry, 37, 3358–3368.
Johnston, S. C., Riddle, S. M., Cohen, R. E., & Hill, C. P. (1999). Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO Journal, 18, 3877–3887.
Komander, D., Reyes-Turcu, F., Licchesi, J. D., Odenwaelder, P., Wilkinson, K. D., & Barford, D. (2009). Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Report, 10, 466–473.
Yao, T., Song, L., Jin, J., Cai, Y., Takahashi, H., Swanson, S. K., et al. (2008). Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Molecular Cell, 31, 909–917.
Yao, T., Song, L., Xu, W., DeMartino, G. N., Florens, L., Swanson, S. K., et al. (2006). Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nature Cell Biology, 8, 994–1002.
Qiu, X. B., Ouyang, S. Y., Li, C. J., Miao, S., Wang, L., & Goldberg, A. L. (2006). hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO Journal, 25, 5742–5753.
Hamazaki, J., Iemura, S., Natsume, T., Yashiroda, H., Tanaka, K., & Murata, S. (2006). A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO Journal, 25, 4524–4536.
Lam, Y. A., Xu, W., DeMartino, G. N., & Cohen, R. E. (1997). Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature, 385, 737–740.
Nishikawa, H., Ooka, S., Sato, K., Arima, K., Okamoto, J., Klevit, R. E., et al. (2004). Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. Journal of Biological Chemistry, 279, 3916–3924.
Morris, J. R., & Solomon, E. (2004). BRCA1:BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Human Molecular Genetics, 13, 807–817.
Wu-Baer, F., Lagrazon, K., Yuan, W., & Baer, R. (2003). The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. Journal of Biological Chemistry, 278, 34743–34746.
Chen, A., Kleiman, F. E., Manley, J. L., Ouchi, T., & Pan, Z. Q. (2002). Autoubiquitination of the BRCA1*BARD1 RING ubiquitin ligase. Journal of Biological Chemistry, 277, 22085–22092.
Mallery, D. L., Vandenberg, C. J., & Hiom, K. (2002). Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. EMBO Journal, 21, 6755–6762.
Starita, L. M., Horwitz, A. A., Keogh, M. C., Ishioka, C., Parvin, J. D., & Chiba, N. (2005). BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. Journal of Biological Chemistry, 280, 24498–24505.
Garcia-Higuera, I., Taniguchi, T., Ganesan, S., Meyn, M. S., Timmers, C., Hejna, J., et al. (2001). Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Molecular Cell, 7, 249–262.
Sato, K., Hayami, R., Wu, W., Nishikawa, T., Nishikawa, H., Okuda, Y., et al. (2004). Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. Journal of Biological Chemistry, 279, 30919–30922.
Eakin, C. M., Maccoss, M. J., Finney, G. L., & Klevit, R. E. (2007). Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proceedings of National Academic Science of United States of America, 104, 5794–5799.
Scheuermann, J. C., de Ayala Alonso, A. G., Oktaba, K., Ly-Hartig, N., McGinty, R. K., Fraterman, S., et al. (2010). Histone H2A deubiquitinase activity of the polycomb repressive complex PR-DUB. Nature, 465, 243–247.
Kaestner, K. H., Knochel, W., & Martinez, D. E. (2000). Unified nomenclature for the winged helix/forkhead transcription factors. Genes and Development, 14, 142–146.
Shi, X., Bowlin, K. M., & Garry, D. J. (2010). Fhl2 interacts with foxk1 and corepresses foxo4 activity in myogenic progenitors. Stem Cells, 28, 462–469.
Meeson, A. P., Shi, X., Alexander, M. S., Williams, R. S., Allen, R. E., Jiang, N., et al. (2007). Sox15 and Fhl3 transcriptionally coactivate Foxk1 and regulate myogenic progenitor cells. EMBO Journal, 26, 1902–1912.
Hawke, T. J., Jiang, N., & Garry, D. J. (2003). Absence of p21CIP rescues myogenic progenitor cell proliferative and regenerative capacity in Foxk1 null mice. Journal of Biological Chemistry, 278, 4015–4020.
Jensen, D. E., & Rauscher, F. J., 3rd. (1999). Defining biochemical functions for the BRCA1 tumor suppressor protein: analysis of the BRCA1 binding protein BAP1. Cancer Letters, 143(Suppl 1), S13–S17.
Angeloni, D. (2007). Molecular analysis of deletions in human chromosome 3p21 and the role of resident cancer genes in disease. Briefings in functional genomics and proteomics, 6, 19–39.
Wysocka, J., Reilly, P. T., & Herr, W. (2001). Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Molecular and Cellular Biology, 21, 3820–3829.
Wilson, A. C., LaMarco, K., Peterson, M. G., & Herr, W. (1993). The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell, 74, 115–125.
Wilson, A. C., Peterson, M. G., & Herr, W. (1995). The HCF repeat is an unusual proteolytic cleavage signal. Genes and Development, 9, 2445–2458.
Goto, H., Motomura, S., Wilson, A. C., Freiman, R. N., Nakabeppu, Y., Fukushima, K., et al. (1997). A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes and Development, 11, 726–737.
Reilly, P. T., Wysocka, J., & Herr, W. (2002). Inactivation of the retinoblastoma protein family can bypass the HCF-1 defect in tsBN67 cell proliferation and cytokinesis. Molecular and Cellular Biology, 22, 6767–6778.
Julien, E., & Herr, W. (2003). Proteolytic processing is necessary to separate and ensure proper cell growth and cytokinesis functions of HCF-1. EMBO Journal, 22, 2360–2369.
Freiman, R. N., & Herr, W. (1997). Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes and Development, 11, 3122–3127.
Lu, R., Yang, P., Padmakumar, S., & Misra, V. (1998). The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, luman, in its interaction with HCF. Journal of Virology, 72, 6291–6297.
Luciano, R. L., & Wilson, A. C. (2003). HCF-1 functions as a coactivator for the zinc finger protein Krox20. Journal of Biological Chemistry, 278, 51116–51124.
Yokoyama, A., Wang, Z., Wysocka, J., Sanyal, M., Aufiero, D. J., Kitabayashi, I., et al. (2004). Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Molecular and Cellular Biology, 24, 5639–5649.
Wysocka, J., Myers, M. P., Laherty, C. D., Eisenman, R. N., & Herr, W. (2003). Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3–K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes and Development, 17, 896–911.
Vogel, J. L., & Kristie, T. M. (2000). The novel coactivator C1 (HCF) coordinates multiprotein enhancer formation and mediates transcription activation by GABP. EMBO Journal, 19, 683–690.
Gunther, M., Laithier, M., & Brison, O. (2000). A set of proteins interacting with transcription factor Sp1 identified in a two-hybrid screening. Molecular and Cellular Biochemistry, 210, 131–142.
Narayanan, A., Ruyechan, W. T., & Kristie, T. M. (2007). The coactivator host cell factor-1 mediates Set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection. Proceedings of National Academic Science of United States of America, 104, 10835–10840.
Scarr, R. B., & Sharp, P. A. (2002). PDCD2 is a negative regulator of HCF-1 (C1). Oncogene, 21, 5245–5254.
Ajuh, P. M., Browne, G. J., Hawkes, N. A., Cohen, P. T., Roberts, S. G., & Lamond, A. I. (2000). Association of a protein phosphatase 1 activity with the human factor C1 (HCF) complex. Nucleic Acids Research, 28, 678–686.
Julien, E., & Herr, W. (2004). A switch in mitotic histone H4 lysine 20 methylation status is linked to M phase defects upon loss of HCF-1. Molecular Cell, 14, 713–725.
DeGregori, J., & Johnson, D. G. (2006). Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Current Molecular Medicine, 6, 739–748.
Blais, A., & Dynlacht, B. D. (2004). Hitting their targets: an emerging picture of E2F and cell cycle control. Current Opinion in Genetics and Development, 14, 527–532.
Cam, H., & Dynlacht, B. D. (2003). Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell, 3, 311–316.
Trimarchi, J. M., & Lees, J. A. (2002). Sibling rivalry in the E2F family. Nature Reviews Molecular Cell Biology, 3, 11–20.
Marti, A., Wirbelauer, C., Scheffner, M., & Krek, W. (1999). Interaction between ubiquitin-protein ligase SCFSKP2 and E2F–1 underlies the regulation of E2F-1 degradation. Nature Cell Biology, 1, 14–19.
Shibutani, S. T., de la Cruz, A. F., Tran, V., Turbyfill, W. J., I. I. I., Reis, T., Edgar, B. A., et al. (2008). Intrinsic negative cell cycle regulation provided by PIP box- and Cul4Cdt2-mediated destruction of E2f1 during S phase. Developmental Cell, 15, 890–900.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eletr, Z.M., Wilkinson, K.D. An Emerging Model for BAP1’s Role in Regulating Cell Cycle Progression. Cell Biochem Biophys 60, 3–11 (2011). https://doi.org/10.1007/s12013-011-9184-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9184-6