Abstract
Mesenchymal stem cells (MSC) have become a promising tool for therapeutic intervention. Their unique features, including self-renewal, multipotency and immunomodulatory properties draw the worldwide attention of researchers and physicians with respect to their application in disease treatment. However, the environment (so-called niche) from which MSCs are isolated may determine their usefulness. Many studies indicated the involvement of MSCs in ageing and disease. In this review, we have focused on how type 2 diabetes (T2D) and metabolic syndrome (MS) affect MSC properties, and thus limit their therapeutic potential. Herein, we mainly focus on apoptosis, autophagy and mitochondria deterioration processes that indirectly affect MSC fate. Based on the data presented, special attention should be paid when considering autologous MSC therapy in T2D or MS treatments, as their therapeutic potential may be restricted.
Similar content being viewed by others
Mesenchymal Stem Cells (MSC)
Progressive obesity, insulin resistance, abnormal cholesterol or triglyceride levels that lead to metabolic syndrome (MS) and finally type 2 diabetes (T2D) are emerging problems in the current endocrinology. As reported by the International Diabetes Federation, 382 million of adults (8.3%) are living with diabetes globally; what is more, in the perspective of next 20 years it is estimated that this number will increase to 592 million [1]. Moreover, type 2 diabetes (T2D) has become the leading cause of death among people under the age of 60. These alarming data are becoming increasingly serious both for medicine and the national health care system. Many strategies have been recently proposed to minimize health-related consequences of metabolic syndrome and diabetes. They involve new drug development, including, e.g., glucagon-like peptide (GLP-1) mimetic, dipeptidyl-peptidase-4 (DPP-4) inhibitors, sodium glucose transporter-2 (SGLT2) inhibitors, but also surgical gastric correction, diet-related therapy, such as calorie restriction and finally mesenchymal stem cells application. Mesenchymal stromal stem cells (MSCs) were first described by Fridenstein and colleges in 1970s [2]. These authors demonstrated that in addition to hematopoietic stem cells (HSCs), also rare, plastic-adherent stromal stem cells resided in the bone marrow that showed the ability to form single colonies, the so-called colony-forming unit fibroblasts (CFU-fs). Fridenstein and colleagues observed that this cell population expanded in culture, but more importantly, they demonstrated that MSCs had the ability to differentiate into mesoderm-derived tissue and played an important role in controlling the hematopoietic niche [3]. Thirty-two years later, Zuk and co-workers described for the first time cell population in adipose tissue termed processed lipoaspirate (PLA) cells, which were isolated from human lipoaspirates and, like MSCs, differentiated towards osteogenic, adipogenic, myogenic, and chondrogenic lineages [4]. These physiological features of MSCs are consistent with the general knowledge regarding the regulation of tissue homeostasis. Since Friedenstein, the knowledge about MSC physiological nature has been extensively investigated in the fields of human and veterinary medicine as well as biology [5,6,7,8,9,10,11,12,13,14]. Unique cytophysiological properties of this stem cell population have led to developing a concept, in which their clinical application is consequently implemented. Both MSCs of bone marrow (BMSCs) and adipose tissue (ASCs) have become the most frequently used sources of progenitor cells in the field of tissue engineering and/or regenerative medicine. This population of stem cells, because of their multipotent character, anabolic activity and immunomodulatory effect as well as the ability to differentiate into insulin-producing cells, is a promising candidate in the field of endocrine disorders, including type 2 diabetes and metabolic syndrome [15,16,17]. Here, we analyze physiological characteristics and deterioration of MSCs in metabolic syndrome and diabetes in the context of their potential clinical application.
MSC and Membrane-Derived Vesicles (MVs)
The pro-regenerative nature of MSCs was excellently explained and described for the first time by Ratajczak and colleagues, who proposed membrane-derived vesicles (MVs) as a carrier for a wide range of growth factors secreted into the intercellular space, thereby enhancing regenerative processes [18,19,20]. Moreover, it has been proposed that MVs could be internalized by the donor cell wall or taken up by neighboring cells and ultimately improve intercellular signaling. Therefore, the improvement of intercellular communication is believed to be the initiator of the regenerative process of damaged cells and tissues [21, 22]. For this reason, MVs in addition to MSCs are strongly considered as the candidate to initiate the regenerative process. What is more, MVs have been detected in the circulation and in organs during various diseases, including diabetes and metabolic syndrome [23]. Patients suffering from those disorders, have different cellular MV patterns and those MVs contribute to the development of diabetic macrovascular and microvascular complications. Changes in MV number and composition may serve as potential biomarkers for diagnostic including diabetes and MS.
Bearing in mind the fact that both MS and T2D are characterized by chronic inflammation, searching for systemic, anti-inflammatory agents seems to be fully reasonable. It is very possible that inflammation is the most important factor leading to β-cell dysfunction and eventually death. Hyperglycemia, in combination with altered lipid metabolism (so-called “glucolipotoxicity”), induces local cytokine production and affect the accumulation of oxidative stress factors. Considering the above fact, MSCs and MVs are promising candidates for an effective delivery of anti-inflammatory agents. It was reported that MSCs exerted an immunomodulatory effect through abundant synthesis and secretion of anti-inflammatory cytokines, such as IL-1Ra, IL-10 or transforming growth factor beta (TGF-β) [15]. However, it is not clear how anti-inflammatory cytokines could be transferred to donor β-cells or whole islets. It seems that this direction is fully rational and may bring future advances in MS and T2D treatment. However, it still needs to be explained whether MSCs or MSC-derived MVs obtained from MS or T2D patient will require pharmacological treatment before clinical application. It was shown that MSCs of obese, MS, and T2D patients suffered from increased apoptosis and limited multipotency [16, 24]. These facts should be considered before both MSCs and MVs will find their application in endocrinology practice.
Aging and Senescence of MSC from MS and Diabetic Individuals
Although MSCs can be found in multiple organs and tissues, e.g., bone marrow, adipose tissue, dental pulp and Wharton’s jelly, the final cell yield after isolation is rather low. This creates a demand for prolonged in vitro culture expansion to millions of cells required for therapy. Time of expansion strongly depends and correlates with donors’ age, genetic makeup and clinical history [25,26,27]. It was shown that aged MSCs suffered from multiple deteriorations that strongly diminished their therapeutic utility [26].
Senescence and aging of MSCs has been well known and described in a number of studies that used MSCs from multiple sources, including dental pulp [28,29,30], adipose tissue [8, 31], bone marrow [26, 32], cord blood [33] and endometrium [34]. Senescence activation pathway was correlated with different stress stimuli, such as oxidative stress [35], heat shock or chemotherapeutic agents [36, 37]. It has been demonstrated that aged MSCs accumulate excessive amount of oxidative stress factors and simultaneously suffer from decreased antioxidative defense. Moreover, they exhibit decreased proliferation rate, higher susceptibility to apoptosis and decreased multilineage differentiation potential, which strongly limits their therapeutic value [8, 11, 31, 38].
It was shown, that MS may affect stem cell pool, as the decreased level of CD34 and endothelial progenitor stem cells was associated with MS progression. In normal conditions, MSCs usually remain in a quiescent and undifferentiated state in their niches (pools). Although “alarms” like proinflammatory cytokines, such as INF-α or IL-6, and growth factors like GM-CSF are able to mobilize MSCs and induce their proliferation and homing [39, 40]. Considering the increased caloric uptake in T2D and MS patients, higher secretion of proinflammatory factors may contribute to constant emission of “alarms” and MSC mobilization [41, 42]. Depletion of MSC pool contributes in turn to irreversible impairment of tissue regeneration.
The proinflammatory environment of adipose tissue negatively affects MSC stemness. It was found that MSCs isolated from EMS (equine metabolic syndrome) individuals suffered from many deteriorations, including decreased proliferation rate, clonogenic potential and increased population doubling time [24]. Moreover, MS affected the expression of MSC surface antigens, as substantial reduction of CD90, CD105 and CD73 levels was observed. Decreased expression of CD90 was also observed by Koci et al. [43], although in patients suffering from T2D. Moreover, a study on the diet-induced obese mice showed decreased CD105 expression, which altered the properties of adult stem cells [44]. Interestingly, MS cells were characterized by increased expression of CD44, a cell surface glycoprotein working as an immune cell receptor involved in inflammatory cell activation. Research conducted by Kodama and colleagues [45,46,47] revealed that CD44 was upregulated in white adipose tissue of obese, diabetic mice and humans. Interestingly, CD44 knockout mice fed a high fat diet, did not develop obesity or T2D. The upregulation of CD44 led to migration and infiltration of activated immune cells, increasing the inflammation in adipose tissue; in addition, it was also confirmed that MS adipose tissue was enriched in macrophages secreting IL-1, IL-6 and TNF-alpha. Moreover, reduced expression of MVs was observed in those cells, which could have contributed to their decreased therapeutic potential and utility. Both MS and T2D strongly affect MSC morphology, including actin cytoskeleton organization. MS cells display typical senescence features, including enlarged nucleus and widely spread cellular body, the so-called “fired egg shape” [24]. The increase in the cell population doubling time and changes in their morphology (larger cell size and structural complexity) are also associated with increased apoptosis of MSCs from obese individuals [48].
Susceptibility to apoptosis and increased senescence are another features of MS and T2D stem cells. The upregulation of p53, p21 and BAX was observed in those cells. Moreover, higher levels of anti-apoptotic protein Bcl-2, which prevents activation of caspase cascade and inhibits the release of cytochrome c from mitochondria were also noted. Accordingly, excessive accumulation of senescence-associated β-galactosidase, increased number of dead cells and decreased Ki67 expression may further contribute to the reduction of MSC stemness. Although in the study conducted by Nawrocka et al. [49], these pathological changes caused by T2D appeared to be abolished by the supplementation with basic fibroblast growth factor (bFGF). The addition of bFGF to culture media significantly improved MSC proliferation and decreased apoptosis rate.
Although stem cells retain their self-renewal abilities both in vivo and in vitro, oxidative, inflammatory environment of adipose tissue may contribute to their lower stemness status. Differentiation capacity of MSCs seems to vary depending on cell source, age and patient health status. Most attention has been paid to osteo- and chondrogenic properties of MSCs, because of their potential use in tissue engineering where scaffolds combined with stem cells are applied in damaged organ regeneration. Although MS and T2D contributes to decreased multipotency of MSCs by generating advanced glycation products (AGEs), oxidative stress and inflammation, which can suppress proliferation, induce apoptosis and increase the production of intracellular reactive oxygen species (ROS). Increased apoptosis and ROS accumulation may be partially responsible for the reduced differentiation potential observed in MS cells. It was found during the osteogenic differentiation process that the expression of bone BMP-2 as well as Coll-1 at the mRNA level was significantly downregulated in MS, whereas the expression of RUNX2, OCN and ALP was comparable with the control group [50]. Similar results were obtained during chondrogenic differentiation, as MS cells presented decreased expression of vimentin, decorin and Sox9 [51]. The study conducted by Wu et al. [44] showed that MSCs from obese donors exhibited increased adipogenic and osteogenic differentiation, but decreased chondrogenic capacity. Similarly, MSCs isolated from diabetic patients showed reduced osteogenic differentiation and chemokine CXCL12 downregulation [43].
Metabolic syndrome strongly affects self-renewal and differentiation capacities of MSCs. Thus, their application in clinical practice requires further research and analysis in the context of their safety. The innovative approach is to “rejuvenate” these cells before their clinical use with different chemicals.
Oxidative Stress, Autophagy and Mitophagy in MSCs
Living cells are continuously exposed to the harmful effect of exogenous or endogenous reactive oxygen species (ROS). These highly reactive molecules, radicals and non-radicals, have the ability to capture electrons from molecules they come in contact with, including proteins and nucleic acids, leading in consequence to cell damage. Moreover, oxidative stress may cause non-specific, post-translational protein modifications, leading to aggregate formation. It is commonly known, that the mitochondrial respiratory chain is the main source of ROS in cells. Complexes I and III are most susceptible to electron leakage, resulting in H2O2 formation. Moreover, in certain circumstances, the electron flux is intensified, as in increased energetic demand during endurance exercises. On the other hand, mitochondrial function may decrease during aging or degenerative and metabolic diseases. Hence it is crucial for cells to have a highly effective oxidative defense. Superoxide dismutase (SOD) and glutathione peroxidases are the most important enzymes involved in free radical scavenging. An efficient antioxidant system is also necessary to cope with reactive nitric species (RNS) generated by the reaction between O2 and nitric oxide (NO) [52]. Similarly to ROS, excessive accumulation of RNS leads to irreversible damage to biomolecules [53].
Mitochondrial ROS production and oxidation of mitochondrial lipids have been shown to play a role in inducing autophagy. Autophagy (or self-eating) was first described by Christian de Duve in 1963 as a lysosome-mediated degradation process for non-essential or damaged cellular constituents [54, 55]. It is an evolutionarily conserved process, in which cells engulf a portion of the cytoplasm and organelles into double-membraned vesicles called autophagosomes, which later fuse with lysosomes for the degradation of enclosed materials [56]. Generation of the pre-autophagosomal structure requires the beclin-1–class III PI3K (phosphoinositide 3-kinase) complex as well as generation and insertion of LC3 (light chain 3)-II into the autophagosomal membrane [57]. Fusion of autophagosomes to lysosomes may be mediated by Rab7 and a lysosomal transmembrane protein, LAMP-2 [58]. Upon autophagosome formation and fusion of its outer membrane with the lysosome membrane, the contents as well as the inner membrane of autophagosomes are degraded to generate amino acids and other cellular building blocks for recycling by the cell. In addition to its clearing function in response to energy or nutrient deficiency, autophagy is also a quality control mechanism for protein and organelles, including mitochondria [59]. Activation of autophagy subsequently leads to damaged proteins and impaired organelle clearance, allowing to maintain cellular homeostasis and remodeling during development. However, the function of autophagy in MSCs is still poorly understood. Available data indicate the importance of autophagy in self-renewal, differentiation and pluripotency of stem cells [60]. Elimination of damaged, dysfunctional proteins and organelles seems to be essential for maintaining pluripotency of adult stem cells during the quiescent state [61].
Besides the main function of energy production, mitochondria are also able to turn on and tune autophagy. Excessive accumulation of ROS leads to impairment of mitochondria structure and function, which in turn triggers a selective process of mitochondria self-removal called mitophagy. It has been proposed that upon nutrient deprivation, mitochondria protect themselves from degradation by promoting fusion and inhibiting fission events. It is only after long-term starvation that mitochondria undergo fragmentation and are eventually removed by mitophagy [62].
The importance of autophagy in MSC physiology has been established in several studies. Research conducted by Sanchez et al. [63] revealed that stromal cells utilized autophagy for survival and secreted anti-apoptotic factors under nutrient-deprived conditions that can occur in solid tumors. It was also demonstrated that the apoptosis of bone marrow MSCs under hypoxia was regulated by autophagy via the AMPK/mTOR pathway [64]. Moreover, Gao and colleagues [65] reported that the level of autophagy regulated CD4+ T cell immunosuppression through MSCs by affecting TGF-β1 secretion, thereby providing a novel method for improving the therapeutic efficacy of MSCs by autophagy activation.
Emerging body of evidence indicates the role of autophagy in the pathogenesis of MS and T2D [66]. High concentration of glucose and reduced insulin sensitivity lead to imbalances in oxidative defense within the cell, resulting in ROS-mediated damage in both disorders. Thus, autophagy disorders cause the accumulation of deteriorated proteins and organelles. It appears that the accumulation of defective mitochondria contributes to the reduced insulin secretion by β-cells [67]. Moreover, autophagy may play a role in maintaining intracellular insulin content by accelerating insulin degradation rate in β-cells [68]. The occurrence of autophagy in diabetic tissues positively correlates with excessive accumulation of ROS, as these two processes are tightly correlated. It has been shown that ROS are produced in various tissues under diabetic conditions [69]. In the diabetic state, hyperglycemia and subsequent ROS production decrease insulin expression and secretion and cause apoptosis [70,71,72]. In addition, the cells are sensitive to ROS due to the relatively low expression of antioxidant enzymes, such as SOD and glutathione peroxidase [73]. Therefore, it is likely that antioxidant supplementation can protect β-cells against glucose toxicity; this notion was supported by the study of Kaneto et al., who demonstrated that antioxidant treatment retained glucose-stimulated insulin secretion and ameliorated glucose tolerance in obese diabetic C57BL/KsJ-db/db mice [74].
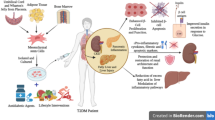
Although ROS and autophagy are being investigated in T2D and MS in various tissues, these processes in diabetic MSCs are still not well explained. It is well known that ROS accumulate with age and their excessive levels contribute to decreased therapeutic potential of aged MSCs [8, 26, 32]. Excessive amounts of ROS in MSCs can impair self-renewal, proliferation and differentiation potential [75, 76]. Thus, it is tempting to speculate that autophagy in MSCs may help to overcome harmful effects of ROS; especially that MS and T2D stem cells are characterized not only by ROS accumulation and decreased SOD activity, but also by mitochondria deterioration [24, 49,50,51]. High frequency of mitochondrial morphological abnormalities, including disarrayed formation of cristae and vacuoles were observed in MSCs isolated from MS individuals. Moreover, PGCα and Parkin expression was significantly decreased in those cells. This fact may contribute to the deterioration of MSMSC osteogenic differentiation, as it has been shown that the transition towards a more oxidative phenotype and increased mitochondrial mass is required to initiate this process [77]. Furthermore, it was reported that excessive ROS inhibited osteogenesis during chondrogenesis and adipogenesis [78]. Hence the hypothesis that ROS and oxidative stress must decrease in order to allow for osteogenic differentiation to proceed seems reasonable. It was shown that MSMSC exhibited decreased osteo- and chondrogenic potential with simultaneous increased rate of mito- and autophagy. The autophagic flux in these cells was probably activated by excessive amount of ROS and damaged mitochondria, which led to nutrient and ATP deprivation. The increased expression of autophagy-related genes, including Beclin-1, LC3 and LAMP2 was observed in MSMSC under controled, chondrogenic and osteogenic conditions. Moreover, p62 concentration was decreased, suggesting that increased autophagy might be a cytoprotective mechanism used by these cells to survive in the inflammatory microenvironment of adipose tissue and to differentiate in vitro. The impaired remodeling of mitochondrial network caused by decreased biogenesis and mitophagy results in the accumulation of damaged mitochondria in MSMSC, which in turn triggers autophagic turnover to generate ATP necessary for effective differentiation [50]. MSC deterioration in metabolic syndrome, including ROS, mitochondria damage and epigenetic alternations is visualized in Fig. 1.
Similar results were obtained during chondrogenic differentiation of MSMSC, as reduced number of mitochondria with a parallel higher expression of lysosomal-associated membrane protein 2 (LAMP2) were observed. Those cells presented slightly impaired chondrogenic differentiation potential, but high auto- and mitophagy allowed to maintain multipotency capacity. Beclin1 and LC3 were downregulated in the native, nonchondrogenic culture, which supports the thesis that autophagy may be a fundamental mechanism that allows MSCs to preserve their multipotency. Moreover, autophagy may be caused by the increased stress of endoplasmic reticulum characteristic of MSMSC [51].
The past decade has witnessed a significant interest in stem cells and autophagy [79, 80]. However, research in these areas is in its infancy. Only few studies have investigated how metabolic syndrome or diabetes affect cytophysiological characteristics of mesenchymal stem cells, such as mitochondria metabolism, autophagy and its role in the differentiation. The potential use of autophagy modulation in optimizing MSC differentiation may improve therapeutic potential of these cells. However, this phenomenon requires further investigation and analysis in the context of MSC safety.
Rejuvenation of Deteriorated cells In Vitro
Searching for effective strategy able to restore stemness and reverse aged phenotype of MSC is crucial while considering their clinical application. Multiple chemicals have been tested in vitro in order to improve viability and differentiation potential of impaired MSC. One of the most effective substance in attenuation of stem cell senescence is resveratrol (RES). It was shown to decrease oxidative stress, reduce apoptosis, enhance proliferation and paracrine activity of MSC [12, 81]. It was also proved, that sirtuin 1 overexpression delays senescence in MSCs that have undergone prolonged culturing and sustain their adipogenic and osteogenic potential [82]. Furthermore, targeting mTOR pathway with rapamycin reversed the senescent phenotype and improved immunoregulation of MSCs [83]. Recent data indicated that algae extract may be beneficial in reversing ROS induced damage and mitochondrial impairment of MSC derived from metabolic syndrome individuals [75]. Authors, have demonstrated that Spirulina platensis extract enhanced viability, suppressed senescence, decreased ROS levels and improved mitochondrial membrane potential of those cells. Furthermore, effectiveness of Spirulina platensis was confirmed in vivo. Algae supplementation decreased weight and improved insulin sensitivity in metabolic syndrome animals. To summarize, application of substances able to reduce oxidative stress in MSCs may in consequence increase the possibility of therapeutic application of this cells.
Concluding Remarks
The age and health of patients strongly affects the status of MSCs. The cells isolated from T2D or MS patients are characterized by increased apoptosis, autophagy, ROS accumulation and mitochondria deterioration. The autophagic flux observed in these cells may be a protective mechanism that provides energy and building blocks to restore cellular homeostasis and control oxidative damage. However, to date, only a few studies have shown the role of autophagy in mesenchymal stem cells in metabolic disorders. Based on presented data, therapeutic application of MSC isolated from MS or T2D patients may be limited due to their dysfunctionality.
References
IDF diabetes atlas - Home (n.d.). Retrieved January 2, 2017, from http://www.diabetesatlas.org/.
Friedenstein, A. J., Chailakhjan, R. K., & Lalykina, K. S. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics, 3(4), 393–403.
Friedenstein, A. J., Chailakhyan, R. K., Latsinik, N. V., Panasyuk, A. F., & Keiliss-Borok, I. V. (1974). Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation, 17(4), 331–340.
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., … Hedrick, M. H. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 13(12), 4279–4295. https://doi.org/10.1091/mbc.E02-02-0105.
Marycz, K., Krzak, J., Urbański, W., Pezowicz, C. (2014). In vitro and in vivo evaluation of sol-gel derived TiO2 coatings based on a variety of precursors and synthesis conditions. Journal of Nanomaterials, 2014, e350579. https://doi.org/10.1155/2014/350579.
Marycz, K., Grzesiak, J., Wrzeszcz, K., & Golonka, P. (2012). Adipose stem cell combined with plasma-based implant bone tissue differentiation in vitro and in a horse with a phalanx digitalis distalis fracture: a case report. Veterinarni Medicina (Czech Republic). Retrieved from http://agris.fao.org/agris-search/search.do?recordID=CZ2013000199.
Grzesiak, J., Krzysztof, M., Karol, W., & Joanna, C. (2011). Isolation and morphological characterisation of ovine adipose-derived mesenchymal stem cells in culture. International Journal of Stem Cells, 4(2), 99–104.
Kornicka, K., Marycz, K., Tomaszewski, K. A., Marędziak, M., & Śmieszek, A. (2015). The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxidative Medicine and Cellular Longevity, 2015, 309169. https://doi.org/10.1155/2015/309169.
Marędziak, M., Marycz, K., Lewandowski, D., Siudzińska, A., & Śmieszek, A. (2015). Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)-a new approach in veterinary regenerative medicine. In Vitro Cellular & Developmental Biology. Animal, 51(3), 230–240. https://doi.org/10.1007/s11626-014-9828-0.
Nicpoń, J., Marycz, K., & Grzesiak, J. (2013). Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Polish Journal of Veterinary Sciences, 16(4), 753–754.
Marędziak, M., Marycz, K., Tomaszewski, K. A., Kornicka, K., & Henry, B. M. (2016). The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells International, 2016, 2152435. https://doi.org/10.1155/2016/2152435.
Kornicka, K., Nawrocka, D., Lis-Bartos, A., Marędziak, M., & Marycz, K. (2017). Polyurethane–polylactide-based material doped with resveratrol decreases senescence and oxidative stress of adipose-derived mesenchymal stromal stem cell (ASCs). RSC Advances, 7(39), 24070–24084. https://doi.org/10.1039/C7RA02334K.
Kornicka, K., Marycz, K., Marędziak, M., Tomaszewski, K. A., & Nicpoń, J. (2017). The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. Journal of Cellular and Molecular Medicine, 21(2), 387–401. https://doi.org/10.1111/jcmm.12972.
Cislo-Pakuluk, A., & Marycz, K. (2017). A promising tool in retina regeneration: current perspectives and challenges when using mesenchymal progenitor stem cells in veterinary and human ophthalmological applications. Stem Cell Reviews, 13(5), 598–602. https://doi.org/10.1007/s12015-017-9750-4.
Abdi, R., Fiorina, P., Adra, C. N., Atkinson, M., & Sayegh, M. H. (2008). Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes, 57(7), 1759–1767. https://doi.org/10.2337/db08-0180.
Sun, Y., Chen, L., Hou, X., Hou, W., Dong, J., Sun, L., … Wang, K. (2007). Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chinese Medical Journal, 120(9), 771–776.
Phadnis, S. M., Ghaskadbi, S. M., Hardikar, A. A., & Bhonde, R. R. (2009). Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. The Review of Diabetic Studies : RDS, 6(4), 260–270. https://doi.org/10.1900/RDS.2009.6.260.
Ratajczak, M. Z. (2008). Microvesicles as immune orchestra conductors. Blood, 111(10), 4832–4833. https://doi.org/10.1182/blood-2008-02-136028.
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A., & Ratajczak, M. Z. (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia, 20(9), 1487–1495. https://doi.org/10.1038/sj.leu.2404296.
Ratajczak, J., Miekus, K., Kucia, M., Zhang, J., Reca, R., Dvorak, P., & Ratajczak, M. Z. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia, 20(5), 847–856. https://doi.org/10.1038/sj.leu.2404132.
Huang, Y.-Z., Xie, H.-Q., Silini, A., Parolini, O., Zhang, Y., Deng, L., & Huang, Y.-C. (2017). Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives. Stem Cell Reviews and Reports, 13(5), 575–586. https://doi.org/10.1007/s12015-017-9753-1.
Collino, F., Pomatto, M., Bruno, S., Lindoso, R. S., Tapparo, M., Sicheng, W., … Camussi, G. (2017). Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Reviews and Reports, 13(2), 226–243. https://doi.org/10.1007/s12015-016-9713-1.
Wang, Y., Chen, L., & Liu, M. (2014). Microvesicles and diabetic complications--novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacologica Sinica, 35(4), 433–443. https://doi.org/10.1038/aps.2013.188.
Marycz, K., Kornicka, K., Basinska, K., & Czyrek, A. (2016). Equine metabolic syndrome affects viability, senescence, and stress factors of equine adipose-derived mesenchymal stromal stem cells: new insight into EqASCs isolated from EMS horses in the context of their aging. Oxidative Medicine and Cellular Longevity, 2016, 1–17. https://doi.org/10.1155/2016/4710326.
Sotiropoulou, P. A., Perez, S. A., Salagianni, M., Baxevanis, C. N., & Papamichail, M. (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells (Dayton, Ohio), 24(2), 462–471. https://doi.org/10.1634/stemcells.2004-0331.
Baker, N., Boyette, L. B., & Tuan, R. S. (2015). Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone, 70, 37–47. https://doi.org/10.1016/j.bone.2014.10.014.
Duggal, S., & Brinchmann, J. E. (2011). Importance of serum source for the in vitro replicative senescence of human bone marrow derived mesenchymal stem cells. Journal of Cellular Physiology, 226(11), 2908–2915. https://doi.org/10.1002/jcp.22637.
Cmielova, J., Havelek, R., Soukup, T., Jiroutová, A., Visek, B., Suchánek, J., … Rezacova, M. (2012). Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. International Journal of Radiation Biology, 88(5), 393–404. https://doi.org/10.3109/09553002.2012.666001.
Muthna, D., Soukup, T., Vavrova, J., Mokry, J., Cmielova, J., Visek, B., … Rezacova, M. (2010). Irradiation of adult human dental pulp stem cells provokes activation of p53, cell cycle arrest, and senescence but not apoptosis. Stem Cells and Development, 19(12), 1855–1862. https://doi.org/10.1089/scd.2009.0449.
Seifrtova, M., Havelek, R., Soukup, T., Filipova, A., Mokry, J., & Rezacova, M. (2013). Mitoxantrone ability to induce premature senescence in human dental pulp stem cells and human dermal fibroblasts. Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society, 64(2), 255–266.
Stolzing, A., & Scutt, A. (2006). Age-related impairment of mesenchymal progenitor cell function. Aging Cell, 5(3), 213–224. https://doi.org/10.1111/j.1474-9726.2006.00213.x.
Stolzing, A., Jones, E., McGonagle, D., & Scutt, A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development, 129(3), 163–173. https://doi.org/10.1016/j.mad.2007.12.002.
Ko, E., Lee, K. Y., & Hwang, D. S. (2012). Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells and Development, 21(11), 1877–1886. https://doi.org/10.1089/scd.2011.0284.
Burova, E., Borodkina, A., Shatrova, A., & Nikolsky, N. (2013). Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxidative Medicine and Cellular Longevity, 2013, 474931. https://doi.org/10.1155/2013/474931.
Kim, J.-S., Kim, E.-J., Kim, H.-J., Yang, J.-Y., Hwang, G.-S., & Kim, C.-W. (2011). Proteomic and metabolomic analysis of H2O2-induced premature senescent human mesenchymal stem cells. Experimental Gerontology, 46(6), 500–510. https://doi.org/10.1016/j.exger.2011.02.012.
Skolekova, S., Matuskova, M., Bohac, M., Toro, L., Durinikova, E., Tyciakova, S., … Kucerova, L. (2016). Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell Communication and Signaling: CCS, 14, 4. https://doi.org/10.1186/s12964-016-0127-0.
Minieri, V., Saviozzi, S., Gambarotta, G., Lo Iacono, M., Accomasso, L., Cibrario Rocchietti, E., … Giachino, C. (2015). Persistent DNA damage-induced premature senescence alters the functional features of human bone marrow mesenchymal stem cells. Journal of Cellular and Molecular Medicine, 19(4), 734–743. https://doi.org/10.1111/jcmm.12387.
Kornicka, K., Babiarczuk, B., Krzak, J., & Marycz, K. (2016). The effect of a sol–gel derived silica coating doped with vitamin E on oxidative stress and senescence of human adipose-derived mesenchymal stem cells (AMSCs). RSC Advances, 6(35), 29524–29537. https://doi.org/10.1039/C6RA00029K.
Hemeda, H., Jakob, M., Ludwig, A.-K., Giebel, B., Lang, S., & Brandau, S. (2010). Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells and Development, 19(5), 693–706. https://doi.org/10.1089/scd.2009.0365.
Mansilla, E., Marín, G. H., Drago, H., Sturla, F., Salas, E., Gardiner, C., … Soratti, C. (2006). Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplantation Proceedings, 38(3), 967–969. https://doi.org/10.1016/j.transproceed.2006.02.053.
Gálvez, B. G., San Martín, N., & Rodríguez, C. (2009). TNF-alpha is required for the attraction of mesenchymal precursors to white adipose tissue in Ob/ob mice. PLoS One, 4(2). https://doi.org/10.1371/journal.pone.0004444.
Rizvi, A. A. (2010). Hypertension, obesity, and inflammation: the complex designs of a deadly trio. Metabolic Syndrome and Related Disorders, 8(4), 287–294. https://doi.org/10.1089/met.2009.0116.
Kočí, Z., Turnovcová, K., Dubský, M., Baranovičová, L., Holáň, V., Chudíčková, M., … Kubinová, S. (2014). Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient’s distal limbs with critical ischemia. Cell Biochemistry and Function, 32(7), 597–604. https://doi.org/10.1002/cbf.3056.
Wu, C.-L., Diekman, B. O., Jain, D., & Guilak, F. (2013). Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. International Journal of Obesity (2005), 37(8), 1079–1087. https://doi.org/10.1038/ijo.2012.171.
Liu, L. F., Kodama, K., Wei, K., Tolentino, L. L., Choi, O., Engleman, E. G., … McLaughlin, T. (2015). The receptor CD44 is associated with systemic insulin resistance and proinflammatory macrophages in human adipose tissue. Diabetologia, 58(7), 1579–1586. https://doi.org/10.1007/s00125-015-3603-y.
Kodama, K., Toda, K., Morinaga, S., Yamada, S., & Butte, A. J. (2015). Anti-CD44 antibody treatment lowers hyperglycemia and improves insulin resistance, adipose inflammation, and hepatic steatosis in diet-induced obese mice. Diabetes, 64(3), 867–875. https://doi.org/10.2337/db14-0149.
Kodama, K., Horikoshi, M., Toda, K., Yamada, S., Hara, K., Irie, J., … Butte, A. J. (2012). Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proceedings of the National Academy of Sciences of the United States of America, 109(18), 7049–7054. https://doi.org/10.1073/pnas.1114513109.
Pérez, L. M., Bernal, A., de Lucas, B., San Martin, N., Mastrangelo, A., García, A., … Gálvez, B. G. (2015). Altered metabolic and stemness capacity of adipose tissue-derived stem cells from obese mouse and human. PloS One, 10(4), e0123397. https://doi.org/10.1371/journal.pone.0123397.
Marycz, K., Śmieszek, A., Grzesiak, J., Donesz-Sikorska, A., & Krzak-Roś, J. (2013). Application of bone marrow and adipose-derived mesenchymal stem cells for testing the biocompatibility of metal-based biomaterials functionalized with ascorbic acid. Biomedical Materials (Bristol, England), 8(6), 065004. https://doi.org/10.1088/1748-6041/8/6/065004.
Marycz, K., Kornicka, K., Marędziak, M., Golonka, P., & Nicpoń, J. (2016). Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. Journal of Cellular and Molecular Medicine, 20(12), 2384–2404. https://doi.org/10.1111/jcmm.12932.
Marycz, K., Kornicka, K., Grzesiak, J., Śmieszek, A., & Szłapka, J. (2016). Macroautophagy and selective mitophagy ameliorate chondrogenic differentiation potential in adipose stem cells of equine metabolic syndrome: new findings in the field of progenitor cells differentiation. Oxidative Medicine and Cellular Longevity, 2016, e3718468. https://doi.org/10.1155/2016/3718468.
Beckman, J. S., & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. The American Journal of Physiology, 271(5 Pt 1), C1424–C1437.
Allen, C. L., & Bayraktutan, U. (2009). Oxidative stress and its role in the pathogenesis of ischaemic stroke. International Journal of Stroke: Official Journal of the International Stroke Society, 4(6), 461–470. https://doi.org/10.1111/j.1747-4949.2009.00387.x.
De Duve, C. (1963). The lysosome. Scientific American, 208, 64–72.
De Duve, C., & Wattiaux, R. (1966). Functions of lysosomes. Annual Review of Physiology, 28, 435–492. https://doi.org/10.1146/annurev.ph.28.030166.002251.
He, C., & Klionsky, D. J. (2009). Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics, 43, 67–93. https://doi.org/10.1146/annurev-genet-102808-114910.
Chen, Y., & Klionsky, D. J. (2011). The regulation of autophagy - unanswered questions. Journal of Cell Science, 124(Pt 2), 161–170. https://doi.org/10.1242/jcs.064576.
Saftig, P., Beertsen, W., & Eskelinen, E.-L. (2008). LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy, 4(4), 510–512.
Mizushima, N., Yamamoto, A., Hatano, M., Kobayashi, Y., Kabeya, Y., Suzuki, K., … Yoshimori, T. (2001). Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. The Journal of Cell Biology, 152(4), 657–668.
Vessoni, A. T., Muotri, A. R., & Okamoto, O. K. (2012). Autophagy in stem cell maintenance and differentiation. Stem Cells and Development, 21(4), 513–520. https://doi.org/10.1089/scd.2011.0526.
Coller, H. A., Sang, L., & Roberts, J. M. (2006). A new description of cellular quiescence. PLoS Biology, 4(3), e83. https://doi.org/10.1371/journal.pbio.0040083.
Rambold, A. S., Kostelecky, B., Elia, N., & Lippincott-Schwartz, J. (2011). Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proceedings of the National Academy of Sciences of the United States of America, 108(25), 10190–10195. https://doi.org/10.1073/pnas.1107402108.
Sanchez, C. G., Penfornis, P., Oskowitz, A. Z., Boonjindasup, A. G., Cai, D. Z., Dhule, S. S., … Pochampally, R. R. (2011). Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis, 32(7), 964–972. https://doi.org/10.1093/carcin/bgr029.
Zhang, Z., Yang, M., Wang, Y., Wang, L., Jin, Z., Ding, L., … Hu, T. (2016). Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biology International, 40(6), 671–685. https://doi.org/10.1002/cbin.10604.
Gao, L., Cen, S., Wang, P., Xie, Z., Liu, Z., Deng, W., et al. (2016). Autophagy improves the immunosuppression of CD4+ T cells by mesenchymal stem cells through transforming growth factor-β1. Stem Cells Translational Medicine. https://doi.org/10.5966/sctm.2015-0420.
Gonzalez, C. D., Lee, M.-S., Marchetti, P., Pietropaolo, M., Towns, R., Vaccaro, M. I., … Wiley, J. W. (2011). The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy, 7(1), 2–11. :https://doi.org/10.4161/auto.7.1.13044.
Jung, H. S., Chung, K. W., Won Kim, J., Kim, J., Komatsu, M., Tanaka, K., … Lee, M.-S. (2008). Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metabolism, 8(4), 318–324. https://doi.org/10.1016/j.cmet.2008.08.013.
Marsh, B. J., Soden, C., Alarcón, C., Wicksteed, B. L., Yaekura, K., Costin, A. J., … Rhodes, C. J. (2007). Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Molecular Endocrinology (Baltimore, Md.), 21(9), 2255–2269. https://doi.org/10.1210/me.2007-0077.
Baynes, J. W., & Thorpe, S. R. (1999). Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes, 48(1), 1–9. https://doi.org/10.2337/diabetes.48.1.1.
Weir, G. C., Laybutt, D. R., Kaneto, H., Bonner-Weir, S., & Sharma, A. (2001). Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes, 50(Suppl 1), S154–S159.
Prentki, M., & Nolan, C. J. (2006). Islet beta cell failure in type 2 diabetes. The Journal of Clinical Investigation, 116(7), 1802–1812. https://doi.org/10.1172/JCI29103.
Poitout, V., & Robertson, R. P. (2002). Minireview: secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology, 143(2), 339–342. https://doi.org/10.1210/endo.143.2.8623.
Lenzen, S., Drinkgern, J., & Tiedge, M. (1996). Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology & Medicine, 20(3), 463–466.
Kaneto, H., Kajimoto, Y., Miyagawa, J., Matsuoka, T., Fujitani, Y., Umayahara, Y., … Hori, M. (1999). Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes, 48(12), 2398–2406.
Nawrocka, D., Kornicka, K., Śmieszek, A., & Marycz, K. (2017). Spirulina platensis improves mitochondrial function impaired by elevated oxidative stress in adipose-derived mesenchymal stromal cells (ASCs) and intestinal epithelial cells (IECs), and enhances insulin sensitivity in equine metabolic syndrome (EMS) horses. Marine Drugs, 15(8). https://doi.org/10.3390/md15080237.
Marycz, K., Michalak, I., Kocherova, I., Marędziak, M., & Weiss, C. (2017). The cladophora glomerata enriched by biosorption process in Cr(III) improves viability, and reduces oxidative stress and apoptosis in equine metabolic syndrome derived adipose mesenchymal stromal stem cells (ASCs) and their extracellular vesicles (MV’s). Marine Drugs, 15(12). https://doi.org/10.3390/md15120385.
Prowse, A. B. J., Chong, F., Elliott, D. A., Elefanty, A. G., Stanley, E. G., Gray, P. P., … Osborne, G. W. (2012). Analysis of mitochondrial function and localisation during human embryonic stem cell differentiation in vitro. PloS One, 7(12), e52214. https://doi.org/10.1371/journal.pone.0052214.
Chen, C.-T., Shih, Y.-R. V., Kuo, T. K., Lee, O. K., & Wei, Y.-H. (2008). Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells, 26(4), 960–968. https://doi.org/10.1634/stemcells.2007-0509.
Galkowski, D., Ratajczak, M. Z., Kocki, J., & Darzynkiewicz, Z. (2017). Of cytometry, stem cells and fountain of youth. Stem Cell Reviews and Reports, 13(4), 465–481. https://doi.org/10.1007/s12015-017-9733-5.
Prathipati, P., Nandi, S. S., & Mishra, P. K. (2017). Stem cell-derived exosomes, autophagy, extracellular matrix turnover, and miRNAs in cardiac regeneration during stem cell therapy. Stem Cell Reviews and Reports, 13(1), 79–91. https://doi.org/10.1007/s12015-016-9696-y.
Lei, L.-T., Chen, J.-B., Zhao, Y.-L., Yang, S.-P., & He, L. (2016). Resveratrol attenuates senescence of adipose-derived mesenchymal stem cells and restores their paracrine effects on promoting insulin secretion of INS-1 cells through Pim-1. European Review for Medical and Pharmacological Sciences, 20(6), 1203–1213.
Yuan, H.-F., Zhai, C., Yan, X.-L., Zhao, D.-D., Wang, J.-X., Zeng, Q., … Pei, X.-T. (2012). SIRT1 is required for long-term growth of human mesenchymal stem cells. Journal of Molecular Medicine, 90(4), 389–400. https://doi.org/10.1007/s00109-011-0825-4.
Gu, Z., Tan, W., Ji, J., Feng, G., Meng, Y., Da, Z., … Cheng, C. (2016). Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging, 8(5), 1102–1114. https://doi.org/10.18632/aging.100925.
Acknowledgements
The publication was supported by the National Science Centre (NCN) grants “Modulation mitochondrial metabolism and dynamics and tageting DNA methylation of adipose derived mesenchymal stromal stem cell (ASC) using resveratrol and 5-azacytydine as a therapeutic strategy in the course of Equine metabolic syndrome (EMS).” (2016/21/B/NZ7/01111) and “The effect of bioactive algae enriched by biosorption on the certain minerals such as Cr(III), Mg(II) and Mn(II) on the status of glucose in the course of metabolic syndrome horses. Evaluation in vitro and in vivo” (2015/18/E/NZ9/00607). The publication was supported by the Wrocław Centre of Biotechnology, the Leading National Research Centre (KNOW) programme for years 2014-2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kornicka, K., Houston, J. & Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev and Rep 14, 337–345 (2018). https://doi.org/10.1007/s12015-018-9809-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9809-x