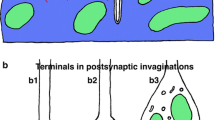
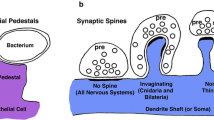
Abstract
Neurons and especially their synapses often project long thin processes that can invaginate neighboring neuronal or glial cells. These “invaginating projections” can occur in almost any combination of postsynaptic, presynaptic, and glial processes. Invaginating projections provide a precise mechanism for one neuron to communicate or exchange material exclusively at a highly localized site on another neuron, e.g., to regulate synaptic plasticity. The best-known types are postsynaptic projections called “spinules” that invaginate into presynaptic terminals. Spinules seem to be most prevalent at large very active synapses. Here, we present a comprehensive review of all kinds of invaginating projections associated with both neurons in general and more specifically with synapses; we describe them in all animals including simple, basal metazoans. These structures may have evolved into more elaborate structures in some higher animal groups exhibiting greater synaptic plasticity. In addition to classic spinules and filopodial invaginations, we describe a variety of lesser-known structures such as amphid microvilli, spinules in giant mossy terminals and en marron/brush synapses, the highly specialized fish retinal spinules, the trophospongium, capitate projections, and fly gnarls, as well as examples in which the entire presynaptic or postsynaptic process is invaginated. These various invaginating projections have evolved to modify the function of a particular synapse, or to channel an effect to one specific synapse or neuron, without affecting those nearby. We discuss how they function in membrane recycling, nourishment, and cell signaling and explore how they might change in aging and disease.









Similar content being viewed by others
References
Acsády, L., Katona, I., Martinez-Guijarro, F. J., Buzsaki, G., & Freund, T. F. (2000). Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. Journal of Neuroscience, 20(18), 6907–6919.
Adamo, N. J., & Daigneault, E. A. (1973a). Ultrastructural features of neurons and nerve fibres in the spiral ganglia of cats. Journal of Neurocytology, 2(1), 91–103.
Adamo, N. J., & Daigneault, E. A. (1973b). Ultrastructural morphology of Schwann cell-neuronal relationships in the spiral ganglia of cats. American Journal of Anatomy, 138(1), 73–77. doi:10.1002/aja.1001380105.
Altman, J. (1971). Coated vesicles and synaptogenesis. A developmental study in the cerebellar cortex of the rat. Brain Research, 30(2), 311–322.
Altman, J. (1993). Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. Journal of Comparative Neurology, 145, 399–464.
Altman, J., & Bayer, S. A. (1997). Development of the cerebellar system in relation to its evolution, structure, and functions. New York: CRC Press.
Anglade, P., Mouatt-Prigent, A., Agid, Y., & Hirsch, E. (1996). Synaptic plasticity in the caudate nucleus of patients with Parkinson’s disease. Neurodegeneration, 5(2), 121–128.
Ashton, F. T., Li, J., & Schad, G. A. (1999). Chemo- and thermosensory neurons: Structure and function in animal parasitic nematodes. Veterinary Parasitology, 84(3–4), 297–316.
Atwood, H. L., Govind, C. K., & Wu, C. F. (1993). Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. Journal of Neurobiology, 24(8), 1008–1024. doi:10.1002/neu.480240803.
Bailey, C. H., Chen, M., Keller, F., & Kandel, E. R. (1992). Serotonin-mediated endocytosis of apCAM: An early step of learning-related synaptic growth in Aplysia. Science, 256(5057), 645–649.
Bailey, C. H., Thompson, E. B., Castellucci, V. F., & Kandel, E. R. (1979). Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. Journal of Neurocytology, 8(4), 415–444.
Biserova, N. M. (2008). Do glial cells exist in the nervous system of parasitic and free-living flatworms? An ultrastructural and immunocytochemical investigation. Acta Biologica Hungarica, 59(Suppl), 209–219. doi:10.1556/ABiol.59.2008.Suppl.30.
Biserova, N. M., Gordeev, I. I., Korneva, J. V., & Salnikova, M. M. (2010). Structure of the glial cells in the nervous system of parasitic and free-living flatworms. Biology Bulletin, 37(3), 277–287.
Blanco, R. E. (1988). Glial cells in peripheral nerves of the cockroach, Periplaneta americana. Tissue and Cell, 20(5), 771–782.
Blanque, A., Repetto, D., Rohlmann, A., Brockhaus, J., Duning, K., Pavenstädt, H., et al. (2015). Deletion of KIBRA, protein expressed in kidney and brain, increases filopodial-like long dendritic spines in neocortical and hippocampal neurons in vivo and in vitro. Frontiers in Neuroanatomy, 9, 13. doi:10.3389/fnana.2015.00013.
Bonga, S. E. W. (1970). Ultrastructure and histochemistry of neurosecretory cells and neurohaemal areas in the pond snail Lymnaea stagnalis. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 108, 190–224.
Boschek, C. B. (1971). On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 118(3), 369–409.
Boyne, A. F., & McLeod, S. (1979). Ultrastructural plasticity in stimulated nerve terminals: Pseudopodial invasions of abutted terminals in Torpedine ray electric organ. Neuroscience, 4(5), 615–624.
Boyne, A. F., & Tarrant, S. B. (1982). Pseudopodial interdigitations between abutted nerve terminals: diffusion traps which occur in several nuclei of the rat limbic system. Journal of Neuroscience, 2(4), 463–469.
Bozhilova-Pastirova, A., & Ovtscharoff, W. (1999). Intramembranous structure of synaptic membranes with special reference to spinules in the rat sensorimotor cortex. European Journal of Neuroscience, 11(5), 1843–1846.
Buchheit, T. E., & Tytell, M. (1992). Transfer of molecules from glia to axon in the squid may be mediated by glial vesicles. Journal of Neurobiology, 23(3), 217–230. doi:10.1002/neu.480230303.
Budziakowski, M. E., & Mettrick, D. F. (1985). Ultrastructural morphology of the neuropile of the cerebral ganglion of Moniliformis moniliformis (Acanthocephala). Journal of Parasitology, 71(1), 75–85.
Bumbarger, D. J., Wijeratne, S., Carter, C., Crum, J., Ellisman, M. H., & Baldwin, J. G. (2009). Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida). Journal of Comparative Neurology, 512(2), 271–281. doi:10.1002/cne.21882.
Cadete-Leite, A., Tavares, M. A., Paula-Barbosa, M. M., & Gray, E. G. (1986). ‘Perforated’ synapses in frontal cortex of chronic alcohol-fed rats. Journal of Submicroscopic Cytology, 18(3), 495–499.
Cagan, R. L., Kramer, H., Hart, A. C., & Zipursky, S. L. (1992). The bride of sevenless and sevenless interaction: Internalization of a transmembrane ligand. Cell, 69(3), 393–399.
Calverley, R. K., & Jones, D. G. (1987). A serial-section study of perforated synapses in rat neocortex. Cell and Tissue Research, 247(3), 565–572.
Campos-Ortega, J. A., & Strausfeld, N. J. (1973). Synaptic connections of intrinsic cells and basket arborizations in the external plexiform layer of the fly’s eye. Brain Research, 59, 119–136.
Carlin, R. K., & Siekevitz, P. (1983). Plasticity in the central nervous system: Do synapses divide? Proceedings of the National Academy of Sciences of the USA, 80(11), 3517–3521.
Carlson, S. D. (1987). Ultrastructure of the arthropod neuroglia and neuropil. In A. P. Gupta (Ed.), Arthropod brain: Its evolution, development, structure, and functions (pp. 323–346). New York: Wiley.
Case, N. M., Gray, E. G., & Young, J. Z. (1972). Ultrastructure and synaptic relations in the optic lobe of the brain of Eledone and Octopus. Journal of Ultrastructure Research, 39(1), 115–123.
Chi, C., & Carlson, S. D. (1976). Close apposition of photoreceptor cell axons in the house fly. Journal of Insect Physiology, 22(8), 1153–1157.
Chicurel, M. E., & Harris, K. M. (1992). Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of Comparative Neurology, 325(2), 169–182. doi:10.1002/cne.903250204.
Chivet, M., Hemming, F., Pernet-Gallay, K., Fraboulet, S., & Sadoul, R. (2012). Emerging role of neuronal exosomes in the central nervous system. Frontiers in Physiology, 3, 145. doi:10.3389/fphys.2012.00145.
Cocucci, E., & Meldolesi, J. (2015). Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends in Cell Biology, 25(6), 364–372.
Cocucci, E., Racchetti, G., & Meldolesi, J. (2009). Shedding microvesicles: Artefacts no more. Trends in Cell Biology, 19(2), 43–51. doi:10.1016/j.tcb.2008.11.003.
Coggeshall, R. E., & Fawcett, D. W. (1964). The fine structure of the central nervous system of the leech, Hirudo medicinalis. Journal of Neurophysiology, 27, 229–289.
Cohen, A. I. (1973). An ultrastructural analysis of the photoreceptors of the squid and their synaptic connections. 3. Photoreceptor terminations in the optic lobes. Journal of Comparative Neurology, 147(3), 399–426. doi:10.1002/cne.901470306.
Curcio, C. A., McNelly, N. A., & Hinds, J. W. (1985). Aging in the rat olfactory system: Relative stability of piriform cortex contrasts with changes in olfactory bulb and olfactory epithelium. Journal of Comparative Neurology, 235(4), 519–528. doi:10.1002/cne.902350409.
Dayel, M. J., Alegado, R. A., Fairclough, S. R., Levin, T. C., Nichols, S. A., McDonald, K., et al. (2011). Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Development Biology, 357(1), 73–82. doi:10.1016/j.ydbio.2011.06.003.
Desmond, N. L., & Levy, W. B. (1983). Synaptic correlates of associative potentiation/depression: An ultrastructural study in the hippocampus. Brain Research, 265(1), 21–30.
Dilly, P. N., Gray, E. G., & Young, J. Z. (1963). Electron microscopy of optic nerves and optic lobes of Octopus and Eledone. Proceedings of the Royal Society of London. Series B: Biological Sciences, 158, 446–456.
Dino, M. R., Nunzi, M. G., Anelli, R., & Mugnaini, E. (2000). Unipolar brush cells of the vestibulocerebellum: Afferents and targets. Progress in Brain Research, 124, 123–137. doi:10.1016/S0079-6123(00)24013-2.
Dirks, P., Tieding, S., Schneider, I., Mey, J., & Weiler, R. (2004). Characterization of retinoic acid neuromodulation in the carp retina. Journal of Neuroscience Research, 78(2), 177–185. doi:10.1002/jnr.20253.
Dyson, S. E., & Jones, D. G. (1984). Synaptic remodelling during development and maturation: Junction differentiation and splitting as a mechanism for modifying connectivity. Brain Research, 315(1), 125–137.
Eckenhoff, M. F., & Pysh, J. J. (1979). Double-walled coated vesicle formation: Evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. Journal of Neurocytology, 8(5), 623–638.
Erisir, A., & Dreusicke, M. (2005). Quantitative morphology and postsynaptic targets of thalamocortical axons in critical period and adult ferret visual cortex. Journal of Comparative Neurology, 485(1), 11–31. doi:10.1002/cne.20507.
Fabian-Fine, R., Verstreken, P., Hiesinger, P. R., Horne, J. A., Kostyleva, R., Zhou, Y., et al. (2003). Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. Journal of Neuroscience, 23(33), 10732–10744.
Fader, C. M., & Colombo, M. I. (2009). Autophagy and multivesicular bodies: Two closely related partners. Cell Death and Differentiation, 16(1), 70–78. doi:10.1038/cdd.2008.168.
Fairchild, C. L., & Barna, M. (2014). Specialized filopodia: At the “tip” of morphogen transport and vertebrate tissue patterning. Current Opinion in Genetics & Development, 27, 67–73.
Familtsev, D. (2013). Synapses, spines, zinc and pathology of Alzheimer’s disease. Louisville, Kentucky: University of Louisville.
Farley, R. D., & Chan, D. J. (1985). The ultrastructure of the cardiac ganglion of the desert scorpion, Paruroctonus mesaensis (Scorpionida: Vaejovidae). J Morph, 184, 231–252.
Fedorenko, G. M., & Uzdensky, A. B. (2009). Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell and Tissue Research, 337(3), 477–490. doi:10.1007/s00441-009-0825-7.
Fiala, J. C., Allwardt, B., & Harris, K. M. (2002). Dendritic spines do not split during hippocampal LTP or maturation. Nature Neuroscience, 5(4), 297–298. doi:10.1038/nn830.
Fiala, J. C., Feinberg, M., Popov, V., & Harris, K. M. (1998). Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. Journal of Neuroscience, 18(21), 8900–8911.
Floris, A., Dino, M., Jacobowitz, D. M., & Mugnaini, E. (1994). The unipolar brush cells of the rat cerebellar cortex and cochlear nucleus are calretinin-positive: A study by light and electron microscopic immunocytochemistry. Anatomy and Embryology (Berl), 189(6), 495–520.
Fox, C. A., Andrade, A. N., Lu Qui, I. J., & Rafols, J. A. (1974). The primate globus pallidus: A Golgi and electron microscopic study. Journal fur Hirnforschung, 15(1), 75–93.
Friedlander, M. J., Martin, K. A. C., & Wassenhove-McCarthy, D. (1991). Effects of monocular visual deprivation on geniculocortical innervation of area 18 in cat. Journal of Neuroscience, 11(10), 3268–3288.
Fritzsch, B., & Straka, H. (2014). Evolution of vertebrate mechanosensory hair cells and inner ears: Toward identifying stimuli that select mutation driven altered morphologies. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 200(1), 5–18. doi:10.1007/s00359-013-0865-z.
Fröhlich, A., & Meinertzhagen, I. A. (1982). Synaptogenesis in the first optic neuropile of the fly’s visual system. Journal of Neurocytology, 11(1), 159–180.
Gad, H., Low, P., Zotova, E., Brodin, L., & Shupliakov, O. (1998). Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron, 21(3), 607–616.
Ganeshina, O., Berry, R. W., Petralia, R. S., Nicholson, D. A., & Geinisman, Y. (2004a). Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience, 125(3), 615–623.
Ganeshina, O., Berry, R. W., Petralia, R. S., Nicholson, D. A., & Geinisman, Y. (2004b). Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. Journal of Comparative Neurology, 468(1), 86–95.
Gao, C., Cao, W., Bao, L., Zuo, W., Xie, G., Cai, T., et al. (2010). Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nature Cell Biology, 12(8), 781–790. doi:10.1038/ncb2082.
Geinisman, Y. (2000). Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cerebral Cortex, 10(10), 952–962.
Geinisman, Y., deToledo-Morrell, L., & Morrell, F. (1994). Comparison of structural synaptic modifications induced by long-term potentiation in the hippocampal dentate gyrus of young adult and aged rats. Annals of the New York Academy of Sciences, 747, 452–466.
Gennaro, J. F, Jr, Nastuk, W. L., & Rutherford, D. T. (1978). Reversible depletion of synaptic vesicles induced by application of high external potassium to the frog neuromuscular junction. Journal of Physiology, 280, 237–247.
Gonobobleva, E., & Maldonado, M. (2009). Choanocyte ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida). Journal of Morphology, 270(5), 615–627. doi:10.1002/jmor.10709.
Gordon, W. C. (1985). Nonconventional interactions between photoreceptor axons in the butterfly lamina ganglionaris. Zeitschrift für Naturforschung, 40c, 460–463.
Gray, E. G. (1961). The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: Light and electron microscope observations. Journal of Anatomy, 95, 345–356.
Greco, V., Hannus, M., & Eaton, S. (2001). Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell, 106(5), 633–645.
Gregory, W. A., Hall, D. H., & Bennett, M. V. (1988). Satellite glial cells penetrate neurosecretory cells to perinuclear position in the goldfish preoptic area. Developmental Brain Research, 44(1), 1–8.
Gurke, S., Barroso, J. F., & Gerdes, H. H. (2008). The art of cellular communication: Tunneling nanotubes bridge the divide. Histochemistry and Cell Biology, 129(5), 539–550. doi:10.1007/s00418-008-0412-0.
Haamedi, S. N., Karten, H. J., & Djamgoz, M. B. (2001). Nerve growth factor induces light adaptive cellular and synaptic plasticity in the outer retina of fish. Journal of Comparative Neurology, 431(4), 397–404.
Halanych, K. M. (2015). The ctenophore lineage is older than sponges? That cannot be right! Or can it? Journal of Experimental Biology, 218(Pt 4), 592–597. doi:10.1242/jeb.111872.
Harreveld, A. V., & Trubatch, J. (1975). Synaptic changes in frog brain after stimulation with potassium chloride. Journal of Neurocytology, 4(1), 33–46.
Hartfelder, K., Hanton, W. K., & Bollenbacher, W. E. (1994). Diapause-dependent changes in prothoracicotropic hormone-producing neurons of the tobacco hornworm, Manduca sexta. Cell and Tissue Research, 277(1), 69–78.
Heuser, J. E., & Reese, T. S. (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. Journal of Cell Biology, 57(2), 315–344.
Holtmann, M., & Thurm, U. (2001). Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa). J Comp Neurol, 432(4), 537–549.
Hoyle, G., Williams, M., & Philips, C. (1986). Functional morphology of insect neuronal cell-surface/glial contacts: The trophospongium. Journal of Comparative Neurology, 246, 113–128.
Huganir, R. L., & Nicoll, R. A. (2013). AMPARs and synaptic plasticity: The last 25 years. Neuron, 80(3), 704–717. doi:10.1016/j.neuron.2013.10.025.
Jia, X. X., Gorczyca, M., & Budnik, V. (1993). Ultrastructure of neuromuscular junctions in Drosophila: Comparison of wild type and mutants with increased excitability. Journal of Neurobiology, 24(8), 1025–1044. doi:10.1002/neu.480240804.
Jones, D. G., & Calverley, R. K. (1991). Perforated and non-perforated synapses in rat neocortex: Three-dimensional reconstructions. Brain Research, 556(2), 247–258.
Jorgensen, E. M. (2014). Animal evolution: Looking for the first nervous system. Current Biology, 24(14), R655–R658. doi:10.1016/j.cub.2014.06.036.
Joshi, P., Benussi, L., Furlan, R., Ghidoni, R., & Verderio, C. (2015). Extracellular Vesicles in Alzheimer’s Disease: Friends or Foes? Focus on Abeta-Vesicle Interaction. International Journal of Molecular Sciences, 16(3), 4800–4813. doi:10.3390/ijms16034800.
Klooster, J., Yazulla, S., & Kamermans, M. (2009). Ultrastructural analysis of the glutamatergic system in the outer plexiform layer of zebrafish retina. Journal of Chemical Neuroanatomy, 37(4), 254–265. doi:10.1016/j.jchemneu.2009.02.004.
Klueg, K. M., & Muskavitch, M. A. (1999). Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: Members of the Notch signalling pathway in Drosophila. Journal of Cell Science, 112(Pt 19), 3289–3297.
Korkut, C., Ataman, B., Ramachandran, P., Ashley, J., Barria, R., Gherbesi, N., et al. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 139(2), 393–404. doi:10.1016/j.cell.2009.07.051.
Korkut, C., Li, Y., Koles, K., Brewer, C., Ashley, J., Yoshihara, M., et al. (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 77(6), 1039–1046. doi:10.1016/j.neuron.2013.01.013.
Kornberg, T. B., & Roy, S. (2014). Cytonemes as specialized signaling filopodia. Development, 141(4), 729–736. doi:10.1242/dev.086223.
Krämer, H., Cagan, R. L., & Zipursky, S. L. (1991). Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature, 352(6332), 207–212. doi:10.1038/352207a0.
Kröger, R. H., & Wagner, H. J. (1996). Horizontal cell spinule dynamics in fish are affected by rearing in monochromatic light. Vision Research, 36(24), 3879–3889.
Larsen, W. J. (1983). Biological implications of gap junction structure, distribution and composition: A review. Tissue and Cell, 15(5), 645–671.
Leise, E. M., & Cloney, R. A. (1982). Chiton integument: Ultrastructure of the sensory hairs of Mopalia muscosa (Mollusca: Polyplacophora). Cell and Tissue Research, 223(1), 43–59.
Leranth, C., & Frotscher, M. (1986). Synaptic connections of cholecystokinin-immunoreactive neurons and terminals in the rat fascia dentata: A combined light and electron microscopic study. Journal of Comparative Neurology, 254(1), 51–64. doi:10.1002/cne.902540105.
Leys, S. P. (2015). Elements of a ‘nervous system’ in sponges. Journal of Experimental Biology, 218(Pt 4), 581–591. doi:10.1242/jeb.110817.
Li, Y. C., Li, Y. N., Cheng, C. X., Sakamoto, H., Kawate, T., Shimada, O., et al. (2005). Subsurface cisterna-lined axonal invaginations and double-walled vesicles at the axonal-myelin sheath interface. Neuroscience Research, 53(3), 298–303. doi:10.1016/j.neures.2005.07.006.
Mäntylä, K., Reuter, M., Halton, D. W., Maule, A. G., Brennan, G. P., Shaw, C., et al. (1998). The nervous system of Procerodes littoralis (Maricola, Tricladida). An ultrastructural and immunoelectron microscopical study. Acta Zoologica, 79(1), 1–8.
Marston, D. J., Dickinson, S., & Nobes, C. D. (2003). Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nature Cell Biology, 5(10), 879–888. doi:10.1038/ncb1044.
Mashanov, V. S., Zueva, O. R., Heinzeller, T., & Dolmatov, I. Y. (2006). Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology, 125, 27–38.
Matsuo, I., Kimura-Yoshida, C., & Shimokawa, K. (2014). Divergent roles of heparan sulfate in regulation of FGF signaling during mammalian embryogenesis. In H. Kondoh & A. Kuroiwa (Eds.), New principles in developmental processes (pp. 239–251). Japan: Springer.
Matthews, G., & Fuchs, P. (2010). The diverse roles of ribbon synapses in sensory neurotransmission. Nature Reviews Neuroscience, 11(12), 812–822. doi:10.1038/nrn2924.
Medvedev, N. I., Dallérac, G., Popov, V. I., Rodriguez Arellano, J. J., Davies, H. A., Kraev, I. V., et al. (2014). Multiple spine boutons are formed after long-lasting LTP in the awake rat. Brain Structure and Function, 219(1), 407–414. doi:10.1007/s00429-012-0488-0.
Meyer, D., Bonhoeffer, T., & Scheuss, V. (2014). Balance and stability of synaptic structures during synaptic plasticity. Neuron, 82(2), 430–443. doi:10.1016/j.neuron.2014.02.031.
Mitchell, N., Petralia, R. S., Currier, D. G., Wang, Y. X., Kim, A., Mattson, M. P., et al. (2012). Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. Journal of Cell Science, 125(Pt 18), 4207–4213. doi:10.1242/jcs.105080.
Mueller, W. A., Hassel, M., & Grealy, M. (2015). Development and reproduction in humans and animal model species. Berlin: Springer.
Mugnaini, E., Floris, A., & Wright-Goss, M. (1994). Extraordinary synapses of the unipolar brush cell: An electron microscopic study in the rat cerebellum. Synapse, 16(4), 284–311. doi:10.1002/syn.890160406.
Mugnaini, E., Osen, K. K., Dahl, A. L., Friedrich, V. L, Jr, & Korte, G. (1980). Fine structure of granule cells and related interneurons (termed Golgi cells) in the cochlear nuclear complex of cat, rat and mouse. Journal of Neurocytology, 9(4), 537–570.
Mugnaini, E., Sekerkova, G., & Martina, M. (2011). The unipolar brush cell: A remarkable neuron finally receiving deserved attention. Brain Research Reviews, 66(1–2), 220–245. doi:10.1016/j.brainresrev.2010.10.001.
Muralidharan-Chari, V., Clancy, J., Plou, C., Romao, M., Chavrier, P., Raposo, G., et al. (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current Biology, 19(22), 1875–1885. doi:10.1016/j.cub.2009.09.059.
Muriel, M. P., Agid, Y., & Hirsch, E. (2001). Plasticity of afferent fibers to striatal neurons bearing D1 dopamine receptors in Parkinson’s disease. Movement Disorders, 16(3), 435–441.
Murphy, D. D., & Andrews, S. B. (2000). Culture models for the study of estradiol-induced synaptic plasticity. Journal of Neurocytology, 29(5–6), 411–417.
Nickel, M. (2010). Evolutionary emergence of synaptic nervous systems: What can we learn from the non-synaptic, nerveless Porifera? Invertebrate Biology, 129(1), 1–16.
Nitsch, C., & Rinne, U. (1981). Large dense-core vesicle exocytosis and membrane recycling in the mossy fibre synapses of the rabbit hippocampus during epileptiform seizures. Journal of Neurocytology, 10(2), 201–209.
Nordlander, R. H., Masnyi, J. A., & Singer, M. (1975). Distribution of ultrastructural tracers in crustacean axons. Journal of Comparative Neurology, 161, 499–514.
Omiya, Y., Uchigashima, M., Konno, K., Yamasaki, M., Miyazaki, T., Yoshida, T., et al. (2015). VGluT3-Expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. Journal of Neuroscience, 35(10), 4215–4228. doi:10.1523/JNEUROSCI.4681-14.2015.
Osborne, M. P. (1967). The fine structure of neuromuscular junctions in the segmental muscles of the blowfly larva. Journal of Insect Physiology, 13, 827–833.
Palacios-Prü, E. L., Palacios, L., & Mendoza, R. V. (1981). Synaptogenetic mechanisms during chick cerebellar cortex development. Journal of Submicroscopic Cytology, 13(2), 145–167.
Palay, S. L., & Chan-Palay, V. (1974). Cerebellar cortex. Cytology and organization. New York: Springer.
Papassotiropoulos, A., Stephan, D. A., Huentelman, M. J., Hoerndli, F. J., Craig, D. W., Pearson, J. V., et al. (2006). Common Kibra alleles are associated with human memory performance. Science, 314(5798), 475–478. doi:10.1126/science.1129837.
Pappas, G. D., & Purpura, D. P. (1961). Fine structure of dendrites in the superficial neocortical neuropil. Experimental Neurology, 4, 507–530.
Paspalas, C. D., Rakic, P., & Goldman-Rakic, P. S. (2006). Internalization of D2 dopamine receptors is clathrin-dependent and select to dendro-axonic appositions in primate prefrontal cortex. European Journal of Neuroscience, 24(5), 1395–1403. doi:10.1111/j.1460-9568.2006.05023.x.
Passey, S., Pellegrin, S., & Mellor, H. (2004). What is in a filopodium? Starfish versus hedgehogs. Biochemical Society Transactions, 32(Pt 6), 1115–1117. doi:10.1042/BST0321115.
Pavans de Ceccatty, M. (1966). Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annales des Sciences Naturelles—Zoologie et Biologie Animale, 8, 577–614.
Pentreath, V. W., Berry, M. S., & Cobb, J. L. (1975). Nerve-ending specializations in the central ganglia of Planorbis corneus. Cell and Tissue Research, 163(1), 99–110.
Pérez-Cruz, C., Delgado, L., Lopez-Iglesias, C., & Mercade, E. (2015). Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS One, 10(1), e0116896. doi:10.1371/journal.pone.0116896.
Petralia, R. S., Mattson, M. P., & Yao, P. J. (2014a). Communication breakdown: The impact of ageing on synapse structure. Ageing Res Rev, 14, 31–42.
Petralia, R. S., Mattson, M. P., & Yao, P. J. (2014b). Aging and longevity in the simplest animals and the quest for immortality. Ageing Research Reviews, 16, 66–82. doi:10.1016/j.arr.2014.05.003.
Petralia, R. S., Schwartz, C. M., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2011). Subcellular localization of patched and smoothened, the receptors for sonic hedgehog signaling, in the hippocampal neuron. Journal of Comparative Neurology, 519(18), 3684–3699. doi:10.1002/cne.22681.
Petralia, R. S., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2012). Subcellular distribution of patched and smoothened in the cerebellar neurons. Cerebellum, 11(4), 972–981. doi:10.1007/s12311-012-0374-6.
Petralia, R. S., & Wenthold, R. J. (1992). Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. Journal of Comparative Neurology, 318(3), 329–354. doi:10.1002/cne.903180309.
Popov, V. I., Kleschevnikov, A. M., Klimenko, O. A., Stewart, M. G., & Belichenko, P. V. (2011). Three-dimensional synaptic ultrastructure in the dentate gyrus and hippocampal area CA3 in the Ts65Dn mouse model of down syndrome. Journal of Comparative Neurology, 519(7), 1338–1354. doi:10.1002/cne.22573.
Popova, E. (2014). Role of dopamine in distal retina. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 200(5), 333–358. doi:10.1007/s00359-014-0906-2.
Prokop, A., & Meinertzhagen, I. A. (2006). Development and structure of synaptic contacts in Drosophila. Seminars in Cell and Developmental Biology, 17(1), 20–30. doi:10.1016/j.semcdb.2005.11.010.
Raikova, O. I., Reuter, M., Jondelius, U., & Gustafsson, M. K. S. (2000). An immunocytochemical and ultrastructural study of the nervous and muscular systems of Xenoturbella westbladi (Bilateria inc. sed.). Zoomorphology, 120, 107–118.
Rajendran, L., Bali, J., Barr, M. M., Court, F. A., Kramer-Albers, E. M., Picou, F., et al. (2014). Emerging roles of extracellular vesicles in the nervous system. Journal of Neuroscience, 34(46), 15482–15489. doi:10.1523/JNEUROSCI.3258-14.2014.
Ramirez-Weber, F. A., & Kornberg, T. B. (1999). Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell, 97(5), 599–607.
Remis, J. P., Wei, D., Gorur, A., Zemla, M., Haraga, J., Allen, S., et al. (2014). Bacterial social networks: Structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environmental Microbiology, 16(2), 598–610. doi:10.1111/1462-2920.12187.
Renard, E., Vacelet, J., Gazave, E., Lapebie, P., Borchiellini, C., & Ereskovsky, A. V. (2009). Origin of the neuro-sensory system: New and expected insights from sponges. Integrative Zoology, 4(3), 294–308. doi:10.1111/j.1749-4877.2009.00167.x.
Richards, D. A., Mateos, J. M., Hugel, S., de Paola, V., Caroni, P., Gahwiler, B. H., et al. (2005). Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proceedings of the National Academy of Sciences of the USA, 102(17), 6166–6171. doi:10.1073/pnas.0501881102.
Ringstad, N., Gad, H., Low, P., Di Paolo, G., Brodin, L., Shupliakov, O., et al. (1999). Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron, 24(1), 143–154.
Risinger, M. A., & Larsen, W. J. (1981). Endocytosis of cell-cell junctions and spontaneous cell disaggregation in a cultured human ovarian adenocarcinoma. (COLO 316). Tissue and Cell, 13(2), 413–430.
Robbins, J. R., Barth, A. I., Marquis, H., de Hostos, E. L., Nelson, W. J., & Theriot, J. A. (1999). Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. Journal of Cell Biology, 146(6), 1333–1350.
Saint Marie, R. L., & Carlson, S. D. (1982). Synaptic vesicle activity in stimulated and unstimulated photoreceptor axons in the housefly. A freeze-fracture study. Journal of Neurocytology, 11(5), 747–761.
Sasaki, S., & Iwata, M. (1995). Synaptic loss in the proximal axon of anterior horn neurons in motor neuron disease. Acta Neuropathologica, 90(2), 170–175.
Sasaki, S., & Iwata, M. (1996). Synaptic loss in anterior horn neurons in lower motor neuron disease. Acta Neuropathologica, 91(4), 416–421.
Sasaki, S., & Iwata, M. (1999). Ultrastructural change of synapses of Betz cells in patients with amyotrophic lateral sclerosis. Neuroscience Letters, 268(1), 29–32.
Satterlie, R. A., & Case, J. F. (1978). Gap junctions suggest epithelial conduction within the comb plates of the ctenophore Pleurobrachia bachei. Cell and Tissue Research, 193(1), 87–91.
Schmidt, A., Hannah, M. J., & Huttner, W. B. (1997). Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. Journal of Cell Biology, 137(2), 445–458.
Schultz, K., Janssen-Bienhold, U., Gundelfinger, E. D., Kreutz, M. R., & Weiler, R. (2004). Calcium-binding protein Caldendrin and CaMKII are localized in spinules of the carp retina. Journal of Comparative Neurology, 479(1), 84–93. doi:10.1002/cne.20314.
Schuster, T., Krug, M., & Wenzel, J. (1990). Spinules in axospinous synapses of the rat dentate gyrus: Changes in density following long-term potentiation. Brain Research, 523(1), 171–174.
Sebé-Pedrós, A., Burkhardt, P., Sanchez-Pons, N., Fairclough, S. R., Lang, B. F., King, N., et al. (2013). Insights into the origin of metazoan filopodia and microvilli. Molecular Biology and Evolution, 30(9), 2013–2023. doi:10.1093/molbev/mst110.
Shaw, S. R., & Meinertzhagen, I. A. (1986). Evolutionary progression at synaptic connections made by identified homologous neurones. Proceedings of the National Academy of Sciences of the USA, 83(20), 7961–7965.
Sherer, N. M., Lehmann, M. J., Jimenez-Soto, L. F., Horensavitz, C., Pypaert, M., & Mothes, W. (2007). Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biology, 9(3), 310–315. doi:10.1038/ncb1544.
Sherer, N. M., & Mothes, W. (2008). Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends in Cell Biology, 18(9), 414–420. doi:10.1016/j.tcb.2008.07.003.
Shupliakov, O., Low, P., Grabs, D., Gad, H., Chen, H., David, C., et al. (1997). Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science, 276(5310), 259–263.
Smith, J. E., Clark, A. W., & Kuster, T. A. (1977). Suppression by elevated calcium of black widow spider venom activity at frog neuromuscular junctions. Journal of Neurocytology, 6(5), 519–539.
Smith, C. L., Varoqueaux, F., Kittelmann, M., Azzam, R. N., Cooper, B., Winters, C. A., et al. (2014). Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current Biology, 24(14), 1565–1572. doi:10.1016/j.cub.2014.05.046.
Sobkowicz, H. M., August, B. K., Slapnick, S. M., & Luthy, D. F. (1998). Terminal dendritic sprouting and reactive synaptogenesis in the postnatal organ of Corti in culture. Journal of Comparative Neurology, 397(2), 213–230.
Sobkowicz, H. M., Rose, J. E., Scott, G. L., & Levenick, C. V. (1986). Distribution of synaptic ribbons in the developing organ of Corti. Journal of Neurocytology, 15(6), 693–714.
Sobkowicz, H. M., Slapnick, S. M., & August, B. K. (1999). Apoptosis of inner hair cells caused by laser ablation of their spiral ganglion neurons in cultures of the mouse organ of Corti. Journal of Neurocytology, 28(10–11), 939–954.
Sobkowicz, H. M., Slapnick, S. M., & August, B. K. (2002). Differentiation of spinous synapses in the mouse organ of corti. Synapse, 45(1), 10–24. doi:10.1002/syn.10080.
Sobkowicz, H. M., Slapnick, S. M., & August, B. K. (2003). Reciprocal synapses between inner hair cell spines and afferent dendrites in the organ of corti of the mouse. Synapse, 50(1), 53–66. doi:10.1002/syn.10241.
Sorra, K. E., Fiala, J. C., & Harris, K. M. (1998). Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. Journal of Comparative Neurology, 398(2), 225–240.
Spacek, J., & Harris, K. M. (2004). Trans-endocytosis via spinules in adult rat hippocampus. Journal of Neuroscience, 24(17), 4233–4241. doi:10.1523/JNEUROSCI.0287-04.2004.
Stark, W. S., & Carlson, S. D. (1986). Ultrastructure of capitate projections in the optic neuropil of Diptera. Cell and Tissue Research, 246(3), 481–486.
Stark, W. S., Sapp, R., & Carlson, S. D. (1989). Ultrastructure of the ocellar visual system in normal and mutant Drosophila melanogaster. Journal of Neurogenetics, 5, 127–153.
Stewart, M. G., Davies, H. A., Sandi, C., Kraev, I. V., Rogachevsky, V. V., Peddie, C. J., et al. (2005a). Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience, 131(1), 43–54. doi:10.1016/j.neuroscience.2004.10.031.
Stewart, M. G., Medvedev, N. I., Popov, V. I., Schoepfer, R., Davies, H. A., Murphy, K., et al. (2005b). Chemically induced long-term potentiation increases the number of perforated and complex postsynaptic densities but does not alter dendritic spine volume in CA1 of adult mouse hippocampal slices. European Journal of Neuroscience, 21(12), 3368–3378. doi:10.1111/j.1460-9568.2005.04174.x.
Sukhdeo, S. C., & Sukhdeo, M. V. K. (1994). Mesenchyme cells in Fasciola hepatica (Platyhelminthes): Primitive glia? Tissue and Cell, 26(1), 123–131.
Tao-Cheng, J. H., Dosemeci, A., Gallant, P. E., Miller, S., Galbraith, J. A., Winters, C. A., et al. (2009). Rapid turnover of spinules at synaptic terminals. Neuroscience, 160(1), 42–50. doi:10.1016/j.neuroscience.2009.02.031.
Tarrant, S. B., & Routtenberg, A. (1977). The synaptic spinule in the dendritic spine: Electron microscopic study of the hippocampal dentate gyrus. Tissue and Cell, 9(3), 461–473.
Tarrant, S. B., & Routtenberg, A. (1979). Postsynaptic membrane and spine apparatus: Proximity in dendritic spines. Neuroscience Letters, 11(3), 289–294.
Toh, Y., & Kuwabara, M. (1974). Fine structure of the dorsal ocellus of the worker honeybee. Journal of Morphology, 143, 285–306.
Toh, Y., & Kuwabara, M. (1975). Synaptic organization of the fleshfly ocellus. Journal of Neurocytology, 4(3), 271–287.
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R., & Muller, D. (1999). LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature, 402(6760), 421–425. doi:10.1038/46574.
Trujillo-Cenóz, O. (1965). Some aspects of the structural organization of the intermediate retina of dipterans. Journal of Ultrastructure Research, 13(1), 1–33.
Ueda, Y. (2014). The role of phosphoinositides in synapse function. Molecular Neurobiology, 50(3), 821–838. doi:10.1007/s12035-014-8768-8.
Ueda, Y., & Hayashi, Y. (2013). PIP(3) regulates spinule formation in dendritic spines during structural long-term potentiation. Journal of Neuroscience, 33(27), 11040–11047. doi:10.1523/JNEUROSCI.3122-12.2013.
Vaughn, J. E. (1989). Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse, 3(3), 255–285. doi:10.1002/syn.890030312.
Wagner, H. J. (1980). Light-dependent plasticity of the morphology of horizontal cell terminals in cone pedicles of fish retinas. Journal of Neurocytology, 9(5), 573–590.
Wagner, H. J., & Djamgoz, M. B. (1993). Spinules: a case for retinal synaptic plasticity. Trends in Neurosciences, 16(6), 201–206.
Wanner, G., Vogl, K., & Overmann, J. (2008). Ultrastructural characterization of the prokaryotic symbiosis in “Chlorochromatium aggregatum”. Journal of Bacteriology, 190(10), 3721–3730. doi:10.1128/JB.00027-08.
Waxman, S. G., Waxman, M., & Pappas, G. D. (1980). Coordinated micropinocytotic activity of adjacent neuronal membranes in mammalian central nervous system. Neuroscience Letters, 20(2), 141–146.
Weedman, D. L., Pongstaporn, T., & Ryugo, D. K. (1996). Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: Mossy fiber endings and their targets. The Journal of Comparative Neurology, 369(3), 345–360. doi:10.1002/(SICI)1096-9861(19960603)369:3<345:AID-CNE2>3.0.CO;2-5.
Weiler, R., & Schultz, K. (1993). Ionotropic non-N-methyl-D-aspartate agonists induce retraction of dendritic spinules from retinal horizontal cells. Proceedings of the National Academy of Sciences of the USA, 90(14), 6533–6537.
Weiler, R., Schultz, K., & Janssen-Bienhold, U. (1996). Ca(2+)-dependency of spinule plasticity at dendrites of retinal horizontal cells and its possible implication for the functional role of spinules. Vision Research, 36(24), 3891–3900.
Westfall, J. A. (1970). Ultrastructure of synapses in a primitive coelenterate. Journal of Ultrastructure Research, 32(3), 237–246.
Westrum, L. E., & Blackstad, T. W. (1962). An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. Journal of Comparative Neurology, 119, 281–309.
Wierenga, C. J., Becker, N., & Bonhoeffer, T. (2008). GABAergic synapses are formed without the involvement of dendritic protrusions. Nature Neuroscience, 11(9), 1044–1052. doi:10.1038/nn.2180.
Williams, J. B. (1994). Unicellular adhesive secretion glands and other cells in the parenchyma of Temnocephala novaezealandiae (Platyhelminthes, Temnocephaloidea): Intercell relationships and nuclear pockets. New Zealand Journal of Zoology, 21(2), 167–178.
Wood, C. R., & Rosenbaum, J. L. (2015). Ciliary ectosomes: Transmissions from the cell’s antenna. Trends in Cell Biology, 25(5), 276–285.
Wright, K. A. (1992). Peripheral sensilla of some lower invertebrates: The Platyhelminthes and Nematoda. Microscopy Research and Technique, 22(3), 285–297. doi:10.1002/jemt.1070220306.
Wright, K. A., & Hui, N. (1976). Post-labial sensory structures on the cecal worm, Heterakis gallinarum. Journal of Parasitology, 62(4), 579–584.
Yao, P. J., Petralia, R. S., Bushlin, I., Wang, Y., & Furukawa, K. (2005). Synaptic distribution of the endocytic accessory proteins AP180 and CALM. Journal of Comparative Neurology, 481(1), 58–69. doi:10.1002/cne.20362.
Yoshida, T., Uchigashima, M., Yamasaki, M., Katona, I., Yamazaki, M., Sakimura, K., et al. (2011). Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences of the USA, 108(7), 3059–3064. doi:10.1073/pnas.1012875108.
Zhao, H. M., Wenthold, R. J., & Petralia, R. S. (1998). Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. Journal of Neuroscience, 18(14), 5517–5528.
Zimmer, J., Lawrence, J., & Raisman, G. (1982). A quantitative electron microscopic study of synaptic reorganization in the rat medial habenular nucleus after transection of the stria medullaris. Neuroscience, 7(8), 1905–1928.
Acknowledgments
This work was supported by the Intramural Research Programs of NIDCD/NIH and NIA/NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petralia, R.S., Wang, YX., Mattson, M.P. et al. Structure, Distribution, and Function of Neuronal/Synaptic Spinules and Related Invaginating Projections. Neuromol Med 17, 211–240 (2015). https://doi.org/10.1007/s12017-015-8358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-015-8358-6