Abstract
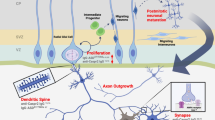
We briefly review the protective role of maternal antibodies during fetal development and at early postnatal stages. We describe antibody delivery to fetuses, particularly in the context of the developing blood–brain barrier (BBB), and present the essential concepts regarding the adult BBB, together with existing information on the prenatal developing BBB. We focus on maternal antibody transfer to the developing brain and the consequences of the presence of pathogenic antibodies at early stages of brain development on subsequent brain dysfunction.



Similar content being viewed by others
References
Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, et al. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182(5):2583–9. doi:10.4049/jimmunol.0803247.
Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi:10.1155/2012/985646.
Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–55.
van den Berg JP, Westerbeek EA, van der Klis FR, Berbers GA, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87(2):67–72. doi:10.1016/j.earlhumdev.2010.11.003.
Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1(6):667–9.
Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442–74. doi:10.3390/nu3040442.
Kobayashi R, Mii S, Nakano T, Harada H, Eto H. Neonatal lupus erythematosus in Japan: a review of the literature. Autoimmun Rev. 2009;8(6):462–6. doi:10.1016/j.autrev.2008.12.013.
Lee LA. Neonatal lupus erythematosus: clinical findings and pathogenesis. J Investig Dermatol Symp Proc. 2004;9(1):52–6. doi:10.1111/j.1087-0024.2004.00827.x.
Motta M, Chirico G, Rebaioli CB, Faden D, Lojacono A, Allegri F, et al. Anticardiolipin and anti-beta2 glycoprotein I antibodies in infants born to mothers with antiphospholipid antibody-positive autoimmune disease: a follow-up study. Am J Perinatol. 2006;23(4):247–51. doi:10.1055/s-2006-939533.
Tincani A, Nuzzo M, Motta M, Zatti S, Lojacono A, Faden D. Autoimmunity and pregnancy: autoantibodies and pregnancy in rheumatic diseases. Ann N Y Acad Sci. 2006;1069:346–52. doi:10.1196/annals.1351.032.
Brucato A. Prevention of congenital heart block in children of SSA-positive mothers. Rheumatology (Oxford). 2008;47(Suppl 3):iii35–7. doi:10.1093/rheumatology/ken153.
Stea EA, Routsias JG, Clancy RM, Buyon JP, Moutsopoulos HM, Tzioufas AG. Anti-La/SSB antiidiotypic antibodies in maternal serum: a marker of low risk for neonatal lupus in an offspring. Arthritis Rheum. 2006;54(7):2228–34. doi:10.1002/art.21954.
Eftekhari P, Roegel JC, Lezoualc'h F, Fischmeister R, Imbs JL, Hoebeke J. Induction of neonatal lupus in pups of mice immunized with synthetic peptides derived from amino acid sequences of the serotoninergic 5-HT4 receptor. Eur J Immunol. 2001;31(2):573–9. doi:10.1002/1521-4141(200102)31:2<573:AID-IMMU573>3.0.CO;2-9.
Kamel R, Garcia S, Lezoualc'h F, Fischmeister R, Muller S, Hoebek J, et al. Immunomodulation by maternal autoantibodies of the fetal serotoninergic 5-HT4 receptor and its consequences in early BALB/c mouse embryonic development. BMC Dev Biol. 2007;7:34. doi:10.1186/1471-213X-7-34.
Buyon JP, Clancy R, Di Donato F, Miranda-Carus ME, Askanase AD, Garcia J, et al. Cardiac 5-HT(4) serotoninergic receptors, 52kD SSA/Ro and autoimmune-associated congenital heart block. J Autoimmun. 2002;19(1–2):79–86.
Kamel R, Eftekhari P, Clancy R, Buyon JP, Hoebeke J. Autoantibodies against the serotoninergic 5-HT4 receptor and congenital heart block: a reassessment. J Autoimmun. 2005;25(1):72–6. doi:10.1016/j.jaut.2005.04.005.
Askanase AD, Izmirly PM, Katholi M, Mumtaz J, Buyon JP. Frequency of neuro-psychiatric dysfunction in anti-SSA/SSB exposed children with and without neonatal lupus. Lupus. 2010;19(3):300–6. doi:10.1177/0961203309354542.
Motta M. Neonates from mother with autoimmune disease. Haematol Rep. 2006;2(10):55–9.
Papazian O. Transient neonatal myasthenia gravis. J Child Neurol. 1992;7(2):135–41.
Parlowsky T, Welzel J, Amagai M, Zillikens D, Wygold T. Neonatal pemphigus vulgaris: IgG4 autoantibodies to desmoglein 3 induce skin blisters in newborns. J Am Acad Dermatol. 2003;48(4):623–5. doi:10.1067/mjd.2003.170.
Tincani A, Rebaioli CB, Andreoli L, Lojacono A, Motta M. Neonatal effects of maternal antiphospholipid syndrome. Curr Rheumatol Rep. 2009;11(1):70–6.
Licht C, Model P, Kribs A, Herkenrath P, Michalk DV, Haupt WF, et al. Transient neonatal myasthenia gravis. Nervenarzt. 2002;73(8):774–8.
Tincani A, Danieli E, Nuzzo M, Scarsil M, Motta M, Cimaz R, et al. Impact of in utero environment on the offspring of lupus patients. Lupus. 2006;15(11):801–7.
Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127(6):1034–42. doi:10.1542/peds.2010-2989.
Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3:e278. doi:10.1038/tp.2013.47.
Braunschweig D, Golub, Koenig CM, Qi L, Pessah IN, Van de Water J, et al. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. J Neuroimmunol. 2012;252(1–2):56–65. doi:10.1016/j.jneuroim.2012.08.002.
Camacho J, Jones K, Miller E, Ariza J, Noctor S, Van de Water J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. doi:10.1016/j.bbr.2014.02.045.
Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53(4):533–7. doi:10.1002/ana.10557.
Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22(6):806–16. doi:10.1016/j.bbi.2007.12.007.
Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. 2009;211(1–2):39–48. doi:10.1016/j.jneuroim.2009.03.011.
Rout UK, Dhossche DM. A pathogenetic model of autism involving Purkinje cell loss through anti-GAD antibodies. Med Hypotheses. 2008;71(2):218–21. doi:10.1016/j.mehy.2007.11.012.
Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13(5):385–96.
McAllister DL, Kaplan BJ, Edworthy SM, Martin L, Crawford SG, Ramsey-Goldman R, et al. The influence of systemic lupus erythematosus on fetal development: cognitive, behavioral, and health trends. J Int Neuropsychol Soc. 1997;3(4):370–6.
Ross G, Sammaritano L, Nass R, Lockshin M. Effects of mothers' autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch Pediatr Adolesc Med. 2003;157(4):397–402. doi:10.1001/archpedi.157.4.397.
Urowitz MB, Gladman DD, MacKinnon A, Ibanez D, Bruto V, Rovet J, et al. Neurocognitive abnormalities in offspring of mothers with systemic lupus erythematosus. Lupus. 2008;17(6):555–60. doi:10.1177/0961203308089326.
Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15(1):91–6. doi:10.1038/nm.1892.
Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58(4):1130–5. doi:10.1002/art.23399.
Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):675–8. doi:10.1002/art.21547.
Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124(2):687–94. doi:10.1542/peds.2008-2445.
Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18(11):1171–7. doi:10.1038/mp.2013.101.
Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case–control study. Arch Pediatr Adolesc Med. 2005;159(2):151–7. doi:10.1001/archpedi.159.2.151.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi:10.1038/nrn1824.
Bauer HC, Bauer H. Neural induction of the blood–brain barrier: still an enigma. Cell Mol Neurobiol. 2000;20(1):13–28.
Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi:10.1124/pr.57.2.4.
Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J Neurochem. 2002;81(1):203–6.
Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114(1–2):168–72.
Morita S, Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res. 2012;349(2):589–603. doi:10.1007/s00441-012-1421-9.
Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi:10.3389/fphar.2012.00046.
Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi:10.1038/nature09513.
Davila D, Thibault K, Fiacco TA, Agulhon C. Recent molecular approaches to understanding astrocyte function in vivo. Front Cell Neurosci. 2013;7:272. doi:10.3389/fncel.2013.00272.
Hoffmann A, Bredno J, Wendland M, Derugin N, Ohara P, Wintermark M. High and low molecular weight fluorescein isothiocyanate (FITC)-dextrans to assess blood–brain barrier disruption: technical considerations. Transl Stroke Res. 2011;2(1):106–11. doi:10.1007/s12975-010-0049-x.
Pentsuk N, van der Laan JW. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol. 2009;86(4):328–44. doi:10.1002/bdrb.20201.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi:10.1126/scitranslmed.3009759.
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106(2):641–6. doi:10.1073/pnas.0805165106.
Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202(4):325–32. doi:10.1002/aja.1002020402.
Shue EH, Carson-Walter EB, Liu Y, Winans BN, Ali ZS, Chen J, et al. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood–brain barrier disruption in rodent models. BMC Neurosci. 2008;9:29. doi:10.1186/1471-2202-9-29.
Bloom O, Cheng KF, He M, Papatheodorou A, Volpe BT, Diamond B, et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc Natl Acad Sci USA. 2011;108(25):10255–9. doi:10.1073/pnas.1103555108.
Acknowledgments
This work is supported by National Institute of Health Grant 1PO1AI073693.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kowal, C., Athanassiou, A., Chen, H. et al. Maternal antibodies and developing blood–brain barrier. Immunol Res 63, 18–25 (2015). https://doi.org/10.1007/s12026-015-8714-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8714-5