Abstract
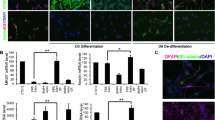
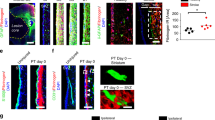
Astrocytes respond to a variety of CNS injuries by cellular enlargement, process outgrowth, and upregulation of extracellular matrix proteins that function to prevent expansion of the injured region. This astrocytic response, though critical to the acute injury response, results in the formation of a glial scar that inhibits neural repair. Scar-forming cells (fibroblasts) in the heart can undergo mesenchymal-endothelial transition into endothelial cell fates following cardiac injury in a process dependent on p53 that can be modulated to augment cardiac repair. Here, we sought to determine whether astrocytes, as the primary scar-forming cell of the CNS, are able to undergo a similar cellular phenotypic transition and adopt endothelial cell fates. Serum deprivation of differentiated astrocytes resulted in a change in cellular morphology and upregulation of endothelial cell marker genes. In a tube formation assay, serum-deprived astrocytes showed a substantial increase in vessel-like morphology that was comparable to human umbilical vein endothelial cells and dependent on p53. RNA sequencing of serum-deprived astrocytes demonstrated an expression profile that mimicked an endothelial rather than astrocyte transcriptome and identified p53 and angiogenic pathways as specifically upregulated. Inhibition of p53 with genetic or pharmacologic strategies inhibited astrocyte-endothelial transition. Astrocyte-endothelial cell transition could also be modulated by miR-194, a microRNA downstream of p53 that affects expression of genes regulating angiogenesis. Together, these studies demonstrate that differentiated astrocytes retain a stimulus-dependent mechanism for cellular transition into an endothelial phenotype that may modulate formation of the glial scar and promote injury-induced angiogenesis.







Similar content being viewed by others
References
Gleichman AJ, Carmichael ST (2014) Astrocytic therapies for neuronal repair in stroke. Neurosci Lett 565:47–52. doi:10.1016/j.neulet.2013.10.055
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50(4):427–34. doi:10.1002/glia.20207
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–47. doi:10.1016/j.tins.2009.08.002
Jones LL, Margolis RU, Tuszynski MH (2003) The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182(2):399–411
Wiese S, Karus M, Faissner A (2012) Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 3:120. doi:10.3389/fphar.2012.00120
Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova-Rainon T et al (2012) Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur J Neurosci 35(8):1208–17. doi:10.1111/j.1460-9568.2012.08041.x
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26(50):13007–16. doi:10.1523/JNEUROSCI.4323-06.2006
Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH (2012) Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A 109(19):7505–10. doi:10.1073/pnas.1121146109
Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW et al (2012) A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A 109(33):E2230–9. doi:10.1073/pnas.1204386109
Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M et al (2011) Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest 121(8):3005–23. doi:10.1172/JCI46358
Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M et al (2012) Neuroprotection from stroke in the absence of MHCI or PirB. Neuron 73(6):1100–7. doi:10.1016/j.neuron.2012.01.020
Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S et al (2014) Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514(7524):585–90. doi:10.1038/nature13839
Obayashi S, Tabunoki H, Kim SU, Satoh J (2009) Gene expression profiling of human neural progenitor cells following the serum-induced astrocyte differentiation. Cell Mol Neurobiol 29(3):423–38. doi:10.1007/s10571-008-9338-2
Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA (2010) The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5(10):e13741. doi:10.1371/journal.pone.0013741
Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5(4):628–35. doi:10.1038/nprot.2010.6
Zudaire E, Gambardella L, Kurcz C, Vermeren S (2011) A computational tool for quantitative analysis of vascular networks. PLoS One 6(11):e27385. doi:10.1371/journal.pone.0027385
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M et al (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. doi:10.1093/bioinformatics/bts635
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31(2):166–9. doi:10.1093/bioinformatics/btu638
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14:128. doi:10.1186/1471-2105-14-128
Zhang Y, Barres, B. (2014) Brain RNAseq. http://web.stanford.edu/group/barres_lab/brain_rnaseq.html.
Busk PK (2014) A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics 15:29. doi:10.1186/1471-2105-15-29
Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X et al (2001) Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294(5549):2186–9. doi:10.1126/science.1065518
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285(5434):1733–7
Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G (2004) Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10(12):1321–8. doi:10.1038/nm1146
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–47. doi:10.1523/JNEUROSCI.1860-14.2014
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol 4(1):1–7
Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Orntoft TF, Andersen CL et al (2008) p53-Responsive microRNAs 192 and 215 are capable of inducing cell cycle arrest. Cancer Res 68(24):10094–104. doi:10.1158/0008-5472.CAN-08-1569
Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q et al (2011) p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res 71(24):7490–501. doi:10.1158/0008-5472.CAN-11-1124
Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF et al (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120(3):421–33. doi:10.1016/j.cell.2004.12.020
Tosh D, Slack JM (2002) How cells change their phenotype. Nat Rev Mol Cell Biol 3(3):187–94. doi:10.1038/nrm761
Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL (2013) In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 15(10):1164–75. doi:10.1038/ncb2843
Su Z, Niu W, Liu ML, Zou Y, Zhang CL (2014) In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 5:3338. doi:10.1038/ncomms4338
Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S et al (2013) Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A 110(17):7038–43. doi:10.1073/pnas.1303829110
Rouaux C, Arlotta P (2013) Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol 15(2):214–21. doi:10.1038/ncb2660
Chen Y, Swanson RA (2003) Astrocytes and brain injury. J Cereb Blood Flow Metab 23(2):137–49
Mi H, Haeberle H, Barres BA (2001) Induction of astrocyte differentiation by endothelial cells. J Neurosci 21(5):1538–47
Ma S, Kwon HJ, Huang Z (2012) A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One 7(10):e48001. doi:10.1371/journal.pone.0048001
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. doi:10.1038/nrn1824
Giaccia AJ, Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev 12(19):2973–83
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10(4):431–42. doi:10.1038/sj.cdd.4401183
Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9(5):402–12. doi:10.1038/nrm2395
Cox LS, Lane DP (1995) Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays 17(6):501–8. doi:10.1002/bies.950170606
Kippin TE, Martens DJ, van der Kooy D (2005) p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev 19(6):756–67. doi:10.1101/gad.1272305
Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J (2006) p53 suppresses the self-renewal of adult neural stem cells. Development 133(2):363–9. doi:10.1242/dev.02208
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G et al (2008) p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 455(7216):1129–33. doi:10.1038/nature07443
Yung HW, Bal-Price AK, Brown GC, Tolkovsky AM (2004) Nitric oxide-induced cell death of cerebrocortical murine astrocytes is mediated through p53- and Bax-dependent pathways. J Neurochem 89(4):812–21. doi:10.1111/j.1471-4159.2004.02395.x
Villapol S, Acarin L, Faiz M, Castellano B, Gonzalez B (2007) Distinct spatial and temporal activation of caspase pathways in neurons and glial cells after excitotoxic damage to the immature rat brain. J Neurosci Res 85(16):3545–56. doi:10.1002/jnr.21450
Salazar JJ, Gallego-Pinazo R, de Hoz R, Pinazo-Duran MD, Rojas B, Ramirez AI, Serrano M, Ramirez JM (2013) “Super p53” mice display retinal astroglial changes. PLoS One 8(6):e65446. doi:10.1371/journal.pone.0065446
Mense SM, Sengupta A, Zhou M, Lan C, Bentsman G, Volsky DJ, Zhang L (2006) Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics 25(3):435–49. doi:10.1152/physiolgenomics.00315.2005
Jebelli JD, Hooper C, Garden GA, Pocock JM (2012) Emerging roles of p53 in glial cell function in health and disease. Glia 60(4):515–25. doi:10.1002/glia.22268
Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S (2011) The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134(Pt 7):2134–48. doi:10.1093/brain/awr142
Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A et al (2013) A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci 33(26):10698–712. doi:10.1523/JNEUROSCI.5772-12.2013
Herdegen T, Delgado-García JM (2004) Brain damage and repair : from molecular research to clinical therapy. Kluwer Academic, Dordrecht
Acknowledgments
We acknowledge the support of the National Institute of Neurological Disorders and Stroke (NINDS) Informatics Center for Neurogenetics and Neurogenomics (P30 NS062691). JDH is supported by (NINDS) NS083740. AD is supported by National Heart, Lung, and Blood Institute (NHLBI) HL129178.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brumm, A.J., Nunez, S., Doroudchi, M.M. et al. Astrocytes Can Adopt Endothelial Cell Fates in a p53-Dependent Manner. Mol Neurobiol 54, 4584–4596 (2017). https://doi.org/10.1007/s12035-016-9974-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9974-3