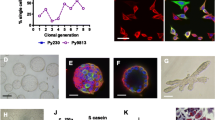
Abstract
Breast cancer stem cells, the root of tumor growth, present challenges to investigate: Primary human breast cancer cells are difficult to establish in culture and inconsistently yield tumors after transplantation into immune-deficient recipient mice. Furthermore, there is limited characterization of mammary cancer stem cells in mice, the ideal model for the study of breast cancer. We herein describe a pre-clinical breast cancer stem cell model, based on the properties of cancer stem cells, derived from transgenic MMTV-PyMT mice. Using a defined set of cell surface markers to identify cancer stem cells by flow cytometry, at least four cell populations were recovered from primary mammary cancers. Only two of the four populations, one epithelial and one mesenchymal, were able to survive and proliferate in vitro. The epithelial population exhibited tumor initiation potential with as few as 10 cells injected into syngeneic immune-competent recipients. Tumors initiated from injected cell lines recapitulated the morphological and physiological components of the primary tumor. To highlight the stemness potential of the putative cancer stem cells, B lymphoma Mo-MLV insertion region 1 homolog (Bmi-1) expression was knocked down via shRNA targeting Bmi-1. Without Bmi-1 expression, putative cancer stem cells could no longer initiate tumors, but tumor initiation was rescued with the introduction of a Bmi-1 overexpression vector in the Bmi-1 knockdown cells. In conclusion, our data show that primary mammary cancers from MMTV-PyMT mice contain putative cancer stem cells that survive in culture and can be used to create a model for study of mammary cancer stem cells.







Similar content being viewed by others
Abbreviations
- Bmi-1:
-
B lymphoma Mo-MLV insertion region 1 homolog
- BSA:
-
Bovine serum albumin
- BrdU:
-
5-bromo-2-deoxyuridine
- CK:
-
Cytokeratin
- CSC:
-
Cancer stem cell
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- EGFP:
-
Enhanced green fluorescent protein
- FACS:
-
Fluorescence-activated cell sorting
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- FMMC:
-
Female mouse mammary cancer cell line
- FVB/NJ:
-
Friend Virus B NIH Jackson mouse strain
- HBSS:
-
Hank’s buffered salt solution
- MCF:
-
Michigan Cancer Foundation cell line
- MMMC:
-
Male mouse mammary cancer cell line
- MMTV-PyMT:
-
Mouse mammary tumor virus promoter-polyoma middle T-antigen
- MSCs:
-
Mesenchymal stem cells
- PBS:
-
Phosphate buffered saline
- RA:
-
All-trans retinoic acid
- SMA:
-
Smooth muscle actin
- TI:
-
Tumor initiation
- TICs:
-
Tumor-initiating cells
References
Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Investig. 1994;70:6–22.
Adriaansen HJ, te Boekhorst PA, Hagemeijer AM, van der Schoot CE, Delwel HR, van Dongen JJ. Acute myeloid leukemia M4 with bone marrow eosinophilia (M4Eo) and inv(16)(p13q22) exhibits a specific immunophenotype with CD2 expression. Blood. 1993;81:3043–51.
Auersperg N, Kruk PA, MacLaren IA, Watt FM, Mydral SE. Heterogeneous expression of keratin, involucrin, and extracellular matrix among subpopulations of a poorly differentiated human cervical carcinoma: possible relationships to patterns of invasion. Cancer Res. 1989;49:3007–14.
Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–12.
Grabowski P, Schonfelder J, Ahnert-Hilger G, Foss HD, Stein H, Berger G, et al. Heterogeneous expression of neuroendocrine marker proteins in human undifferentiated carcinoma of the colon and rectum. Ann N Y Acad Sci. 2004;1014:270–4.
Sutherland HJ, Blair A, Zapf RW. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87:4754–61.
Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73:463–73.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–71.
Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–64.
Hotfilder M, Rottgers S, Rosemann A, Schrauder A, Schrappe M, Pieters R, et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34 + CD19- cells. Cancer Res. 2005;65:1442–9.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–6.
Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–6.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401.
Sell S. Potential gene therapy strategies for cancer stem cells. Curr Gene Ther. 2006;6:579–91.
Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902.
Sell S, Glinsky GV. Preventive and therapeutic strategies for cancer stem cells. In: Farrar W, editor. Cancer stem cells. Cambridge: Cambridge University Press; 2010. p. 68–92.
Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501.
Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3.
Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61.
Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–60.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71.
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7.
Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5.
Raaphorst FM. Self-renewal of hematopoietic and leukemic stem cells: a central role for the Polycomb-group gene Bmi-1. Trends Immunol. 2003;24:522–4.
Park I-K, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–9.
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon SK, Joo JH, et al. Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast. 2004;13:383–8.
Pietersen AM, Evers B, Prasad AA, Tanger E, Cornelissen-Steijger P, Jonkers J, et al. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol. 2008;18:1094–9.
Saeki M, Kobayashi D, Tsuji N, Kuribayashi K, Watanabe N. Diagnostic importance of overexpression of Bmi-1 mRNA in early breast cancers. Int J Oncol. 2009;35:511–5.
Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, et al. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10:10.
Riis ML, Luders T, Nesbakken AJ, Vollan HS, Kristensen V, Bukholm IR. Expression of BMI-1 and Mel-18 in breast tissue—a diagnostic marker in patients with breast cancer. BMC Cancer. 2010;10:686.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7.
Buggiano V, Schere-Levy C, Abe K, Vanzulli S, Piazzon I, Smith GH, et al. Impairment of mammary lobular development induced by expression of TGFbeta1 under the control of WAP promoter does not suppress tumorigenesis in MMTV-infected transgenic mice. Int J Cancer. 2001;92:568–76.
Marquardt D. An algorithm for least-squares estimation of nonlinear parameters. SIAM J Appl Math. 1963;11:431–41.
Bishop YM, Fienberg SE, Holland PW. Discrete multivariate analysis: theory and practice. New York: Springer Science + Business Media; 2007.
Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7.
Feng B, Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother Radiopharm. 2009;24:717–21.
Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–91.
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59–72.
Reinhard MC, Goltz HL, Warner SG, Mirand EA. Growth rate and percentage takes following inoculation of known numbers of viable mouse tumor cells. Exp Med Surg. 1952;10:254–8.
Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 1998;95:3902–7.
Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–9.
Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 2000;97:10960–5.
Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J, Xia S. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene. 2011.
Redova M, Chlapek P, Loja T, Zitterbart K, Hermanova M, Sterba J, et al. Influence of LOX/COX inhibitors on cell differentiation induced by all-trans retinoic acid in neuroblastoma cell lines. Int J Mol Med. 2010;25:271–80.
Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A. Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer. 2009;125:1710–20.
Li RJ, Ying X, Zhang Y, Ju RJ, Wang XX, Yao HJ, et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J Control Release. 2011;149:281–91.
Levkoff LH, Marshall 2nd GP, Ross HH, Caldeira M, Reynolds BA, Cakiroglu M, et al. Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia. 2008;10:804–16.
Ross HH, Levkoff LH, Marshall 2nd GP, Caldeira M, Steindler DA, Reynolds BA, et al. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells. 2008;26:3218–27.
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8.
Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7.
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9.
Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene. 2000;19:992–1001.
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–88.
Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–305.
Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–21.
Wang Q, Li WL, You P, Su J, Zhu MH, Xie DF, et al. Oncoprotein BMI-1 induces the malignant transformation of HaCaT cells. J Cell Biochem. 2009;106:16–24.
Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–93.
Cui H, Hu B, Li T, Ma J, Alam G, Gunning WT, et al. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am J Pathol. 2007;170:1370–8.
Jagani Z, Wiederschain D, Loo A, He D, Mosher R, Fordjour P, et al. The Polycomb group protein Bmi-1 is essential for the growth of multiple myeloma cells. Cancer Res. 2010;70:5528–38.
Acknowledgments
The authors would like to thank Andrew Reilly for his statistical analysis of the TI data.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by NIH R01 CA 112481 (Dr. Sell) and the Ordway Research Institute (Dr. Glinsky).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, J., Lanza, D.G., Guest, I. et al. Characterization of mammary cancer stem cells in the MMTV-PyMT mouse model. Tumor Biol. 33, 1983–1996 (2012). https://doi.org/10.1007/s13277-012-0458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0458-4