Abstract
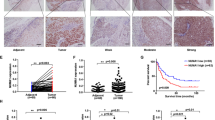
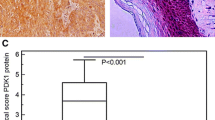
Protein kinase C iota (PKCι) has been shown to play an important role in tumorigenesis of many cancers. It was reported that frequent amplification and overexpression of PKCi were correlated with resistance to anoikis in primary esophageal squamous cell carcinomas (ESCC). In this study, we clarified a novel role of PKCι on the cell cycle progression and proliferation in ESCC. Western blot and immunohistochemistry (IHC) analysis showed that the expression of PKCι was higher in ESCC tumor tissues and cell lines. Meanwhile, IHC stain revealed that PKCι was positively correlated with clinical pathologic variables such as tumor size, tumor grade, and tumor invasion, as well as ki67. Immunoprecipitation and immunofluorescence assay revealed that PKCι/CDK7 has the physical interaction and were co-located in the cell nucleus. And this direct interaction could increase the phosphorylation level of CDK7. In vitro studies such as starvation and refeeding assay along with PKCι-shRNA transfection assay demonstrated that PKCι expression promoted proliferation of ESCC cells. And knocking PKCi down by silencing RNA (siRNA) significantly caused cell cycle arrest at G0/G1 phase, decreased rate of colony formation, and alleviated cellular apoptosis. This research provide new insights into PKCi signaling to more deeply understand its cancer-promoting function in ESCC.






Similar content being viewed by others
References
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–509. doi:10.1056/NEJMra1314530.
Wu C, Wang Z, Song X, Feng XS, Abnet CC, He J, et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet. 2014;46(9):1001–6. doi:10.1038/ng.3064.
Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230(3):392–400 .discussion -3
Zhang J, Anastasiadis PZ, Liu Y, Thompson EA, Fields AP. Protein kinase C (PKC) betaII induces cell invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling pathway. J Biol Chem. 2004;279(21):22118–23. doi:10.1074/jbc.M400774200.
Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252(21):7610–6.
Kishimoto A, Takai Y, Mori T, Kikkawa U, Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980;255(6):2273–6.
Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta ) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000;275(27):20806–13. doi:10.1074/jbc.M000421200.
Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25(2):139–51. doi:10.1016/j.ccr.2014.01.008.
Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010;70(5):2064–74. doi:10.1158/0008-5472.CAN-09-2684.
Rosse C, Lodillinsky C, Fuhrmann L, Nourieh M, Monteiro P, Irondelle M, et al. Control of MT1-MMP transport by atypical PKC during breast-cancer progression. Proc Natl Acad Sci U S A. 2014;111(18):E1872–9. doi:10.1073/pnas.1400749111.
Wang Y, Wang Y, Xiang J, Ji F, Deng Y, Tang C, et al. Knockdown of CRM1 inhibits the nuclear export of p27(Kip1) phosphorylated at serine 10 and plays a role in the pathogenesis of epithelial ovarian cancer. Cancer Lett. 2014;343(1):6–13. doi:10.1016/j.canlet.2013.09.002.
Meng Y, Zhang C, Zhou X. Association between the cyclin D1 G870 A polymorphism and the susceptibility to and prognosis of upper aerodigestive tract squamous cell carcinomas: an updated meta-analysis. Onco Targets Ther. 2016;9:367–76. doi:10.2147/OTT.S94635.
Desai SR, Pillai PP, Patel RS, McCray AN, Win-Piazza HY, Acevedo-Duncan ME. Regulation of Cdk7 activity through a phosphatidylinositol (3)-kinase/PKC-iota-mediated signaling cascade in glioblastoma. Carcinogenesis. 2012;33(1):10–9. doi:10.1093/carcin/bgr231.
Nanos-Webb A, Bui T, Karakas C, Zhang D, Carey JP, Mills GB, et al. PKCiota promotes ovarian tumor progression through deregulation of cyclin E. Oncogene. 2015. doi:10.1038/onc.2015.301.
Bisteau X, Paternot S, Colleoni B, Ecker K, Coulonval K, De Groote P, et al. CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genet. 2013;9(5):e1003546. doi:10.1371/journal.pgen.1003546.
Devos M, Mommaerts E, Migeot V, van Bakel H, Hermand D. Fission yeast Cdk7 controls gene expression through both its CAK and C-terminal domain kinase activities. Mol Cell Biol. 2015;35(9):1480–90. doi:10.1128/MCB.00024-15.
Wang Y, Liu F, Mao F, Hang Q, Huang X, He S, et al. Interaction with cyclin H/cyclin-dependent kinase 7 (CCNH/CDK7) stabilizes C-terminal binding protein 2 (CtBP2) and promotes cancer cell migration. J Biol Chem. 2013;288(13):9028–34. doi:10.1074/jbc.M112.432005.
Franco J, Balaji U, Freinkman E, Witkiewicz AK, Knudsen ES. Metabolic reprogramming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Rep. 2016. doi:10.1016/j.celrep.2015.12.094.
Ou L, Ferreira AM, Otieno S, Xiao L, Bashford D, Kriwacki RW. Incomplete folding upon binding mediates Cdk4/cyclin D complex activation by tyrosine phosphorylation of inhibitor p27 protein. J Biol Chem. 2011;286(34):30142–51. doi:10.1074/jbc.M111.244095.
Zheng Y, Stamminger T, Hearing P. E2F/Rb family proteins mediate interferon induced repression of adenovirus immediate early transcription to promote persistent viral infection. PLoS Pathog 2016;12(1):e1005415. doi:10.1371/journal.ppat.1005415.
Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014;3. doi:10.7554/eLife.02872.
Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, et al. EGF or PDGF receptors activate atypical PKClambda through phosphatidylinositol 3-kinase. EMBO J. 1996;15(4):788–98.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 81171140, no. 81472272).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Sujie Ni and Lingling Chen contributed equally to this work.
Electronic supplementary material
Fig. S1
Silencing of PKCι in ECA 109 cells. ECA 109 cells were transfected with shRNA targeting either four PKCι or a scrambled sequence (control shRNA) for 48 h. Western blot analysis was performed to evaluate the expression of PKCι. The data showed the PKCι protein levels evidently reduced both in ECA109 cells following PKCι-shRNA#4 when compared with control shRNA transfected cells. (JPEG 4 kb)
Fig. S2
Immunofluorescence assay was again performed to determine the Cellular localization of PKCι with CDK7 after expression of PKCι was resuced. Eca109 cells were transfected with PKCι-shRNA#4 for 48 h. Then, Immunofluorescence assay was again performed The result showed that the expression of aPKCi was obviously reduced, which especially in the nuclear, resulting in few co-localization within aPKCi and CDK7. (JPEG 15 kb)
Rights and permissions
About this article
Cite this article
Ni, S., Chen, L., Li, M. et al. PKC iota promotes cellular proliferation by accelerated G1/S transition via interaction with CDK7 in esophageal squamous cell carcinoma. Tumor Biol. 37, 13799–13809 (2016). https://doi.org/10.1007/s13277-016-5193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5193-9