Abstract
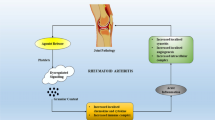
Rheumatoid arthritis (RA) is a chronic autoimmune disease in which imbalances in pro- and anti-inflammatory cytokines promote the induction of autoimmunity, inflammation and joint destruction. Methotrexate, the standard disease-modifying anti-rheumatic drug (DMARD), has shown a gradual loss of efficacy in a significant proportion of patients, probably due to the onset of drug resistance, and thus it was hoped that the development of biologics would revolutionise RA management. Even though biologics have improved the therapy of patients refractive to DMARDs, they require parenteral administration and may leave patients open to serious infection and cancer. Therefore, attention has also been focused on inhibitors of mitogen-activated protein kinases (MAPKs), signalling enzymes that play key roles in pathogenic cytokine production, and their downstream effector pathways, in order to create safe and effective oral drugs. This article therefore provides an overview of the structure and function of MAPKs and their role in the pathogenesis of RA as context to describing the advances in the development of specific, druggable MAPK inhibitors. Their potential as therapies in the management of RA is also discussed.




Similar content being viewed by others
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19.
McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42.
Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–45.
Cai L, Yin JP, Starovasnik MA, Hogue DA, Hillan KJ, Mort JS, et al. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001;16(1):10–21.
Muller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1(2):102–10.
Zhou FH, Foster BK, Zhou XF, Cowin AJ, Xian CJ. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 2006;21(7):1075–88.
Thompson RN, Watts C, Edelman J, Esdaile J, Russell AS. A controlled two-centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. J Rheumatol. 1984;11(6):760–3.
Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312(13):818–22.
Williams HJ, Willkens RF, Samuelson CO Jr, Alarcon GS, Guttadauria M, Yarboro C, et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis: a controlled clinical trial. Arthr Rheum. 1985;28(7):721–30.
Morgan C, Lunt M, Brightwell H, Bradburn P, Fallow W, Lay M, et al. Contribution of patient related differences to multidrug resistance in rheumatoid arthritis. Ann Rheum Dis. 2003;62(1):15–9.
Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012;91(1):30–43.
Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011;70(5):727–32.
Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2(9):717–26.
Cohen S. Small molecular therapies for rheumatoid arthritis: where do we stand? Expert Opin Investig Drugs. 2012;21(1):23–31.
Liu JO. The yins of T cell activation. Sci STKE. 2005;4(265):re1.
Harnett MM, Katz E, Ford CA. Differential signalling during B-cell maturation. Immunol Lett. 2005;98(1):33–44.
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–76.
Goodridge HS, Harnett W, Liew FY, Harnett MM. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology. 2003;109(3):415–25.
Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163(12):6403–12.
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–46.
Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72.
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–83.
Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90(5):859–69.
Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26(22):3240–53.
Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372(Pt 1):1–13.
Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15(5):455–62.
Akella R, Moon TM, Goldsmith EJ. Unique MAP Kinase binding sites. Biochim Biophys Acta. 2008;1784(1):48–55.
Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102(12):4639–750.
Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118.
Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–12.
Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7(11):2135–48.
Ortega-Perez I, Cano E, Were F, Villar M, Vazquez J, Redondo JM. c-Jun N-terminal kinase (JNK) positively regulates NFATc2 transactivation through phosphorylation within the N-terminal regulatory domain. J Biol Chem. 2005;280(21):20867–78.
Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14(23):5957–64.
van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14(8):1798–811.
Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14(20):4905–13.
Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA. 1998;95(18):10541–6.
Lu G, Kang YJ, Han J, Herschman HR, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281(9):6087–95.
Saxena M, Mustelin T. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin Immunol. 2000;12(4):387–96.
Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277(35):31818–25.
Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14(1):6–16.
Masuda K, Shima H, Watanabe M, Kikuchi K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J Biol Chem. 2001;276(42):39002–11.
Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J Biol Chem. 2004;279(40):41882–91.
Jeffrey KL, Brummer T, Rolph MS, Liu SM, Callejas NA, Grumont RJ, et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol. 2006;7(3):274–83.
Fransen J, van Riel PL. Outcome measures in inflammatory rheumatic diseases. Arthr Res Ther. 2009;11(5):244.
Nah SS, Won HJ, Ha E, Kang I, Cho HY, Hur SJ, et al. Epidermal growth factor increases prostaglandin E2 production via ERK1/2 MAPK and NF-kappaB pathway in fibroblast like synoviocytes from patients with rheumatoid arthritis. Rheumatol Int. 2010;30(4):443–9.
Yoo JK, Kwon H, Khil LY, Zhang L, Jun HS, Yoon JW. IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J Immunol. 2005;175(12):8280–6.
Kim YG, Lee CK, Kim SH, Cho WS, Mun SH, Yoo B. Interleukin-32gamma enhances the production of IL-6 and IL-8 in fibroblast-like synoviocytes via Erk1/2 activation. J Clin Immunol. 2010;30(2):260–7.
Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthr Rheum. 2000;43(11):2501–12.
Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1(1):93–103.
Barchowsky A, Frleta D, Vincenti MP. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine. 2000;12(10):1469–79.
Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108(1):73–81.
Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, et al. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthr Rheum. 2003;48(6):1582–93.
Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288(1):208–17.
Thiel MJ, Schaefer CJ, Lesch ME, Mobley JL, Dudley DT, Tecle H, et al. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: potential proinflammatory mechanisms. Arthr Rheum. 2007;56(10):3347–57.
Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand CM, et al. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol. 2009;183(12):8258–67.
Lindstrom TM, Robinson WH. A multitude of kinases: which are the best targets in treating rheumatoid arthritis? Rheum Dis Clin North Am. 2010;36(2):367–83.
Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336(1):357–63.
Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect. 2008;21(5):245–50.
Ohori M, Takeuchi M, Maruki R, Nakajima H, Miyake H. FR180204, a novel and selective inhibitor of extracellular signal-regulated kinase, ameliorates collagen-induced arthritis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(4):311–6.
Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du K, et al. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280(25):23748–57.
Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–83.
Hall JP, Kurdi Y, Hsu S, Cuozzo J, Liu J, Telliez JB, et al. Pharmacologic inhibition of tpl2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J Biol Chem. 2007;282(46):33295–304.
Green N, Hu Y, Janz K, Li HQ, Kaila N, Guler S, et al. Inhibitors of tumor progression loci-2 (Tpl2) kinase and tumor necrosis factor alpha (TNF-alpha) production: selectivity and in vivo antiinflammatory activity of novel 8-substituted-4-anilino-6-aminoquinoline-3-carbonitriles. J Med Chem. 2007;50(19):4728–45.
Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21(3):251–62.
Sundarrajan M, Boyle DL, Chabaud-Riou M, Hammaker D, Firestein GS. Expression of the MAPK kinases MKK-4 and MKK-7 in rheumatoid arthritis and their role as key regulators of JNK. Arthr Rheum. 2003;48(9):2450–60.
Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthr Rheum. 2006;54(7):2127–35.
Lee SI, Boyle DL, Berdeja A, Firestein GS. Regulation of inflammatory arthritis by the upstream kinase mitogen activated protein kinase kinase 7 in the c-Jun N-terminal kinase pathway. Arthr Res Ther. 2012;14(1):R38.
Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM, et al. CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem. 1999;73(5):1901–12.
Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, et al. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem. 2001;276(27):25302–8.
Cui J, Zhang M, Zhang YQ, Xu ZH. JNK pathway: diseases and therapeutic potential. Acta Pharmacol Sin. 2007;28(5):601–8.
Wang W, Ma C, Mao Z, Li M. JNK inhibition as a potential strategy in treating Parkinson’s disease. Drug News Perspect. 2004;17(10):646–54.
Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98(24):13681–6.
Ruckle T, Biamonte M, Grippi-Vallotton T, Arkinstall S, Cambet Y, Camps M, et al. Design, synthesis, and biological activity of novel, potent, and selective (benzoylaminomethyl)thiophene sulfonamide inhibitors of c-Jun-N-terminal kinase. J Med Chem. 2004;47(27):6921–34.
Gaillard P, Jeanclaude-Etter I, Ardissone V, Arkinstall S, Cambet Y, Camps M, et al. Design and synthesis of the first generation of novel potent, selective, and in vivo active (benzothiazol-2-yl)acetonitrile inhibitors of the c-Jun N-terminal kinase. J Med Chem. 2005;48(14):4596–607.
Denninger K, Rasmussen S, Larsen JM, Orskov C, Seier Poulsen S, Sorensen P, et al. JNK1, but not JNK2, is required in two mechanistically distinct models of inflammatory arthritis. Am J Pathol. 2011;179(4):1884–93.
Koller M, Hayer S, Redlich K, Ricci R, David JP, Steiner G, et al. JNK1 is not essential for TNF-mediated joint disease. Arthr Res Ther. 2005;7(1):R166–73.
Gee E, Milkiewicz M, Haas TL. p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J Cell Physiol. 2010;222(1):120–6.
Rousseau D, Cannella D, Boulaire J, Fitzgerald P, Fotedar A, Fotedar R. Growth inhibition by CDK-cyclin and PCNA binding domains of p21 occurs by distinct mechanisms and is regulated by ubiquitin-proteasome pathway. Oncogene. 1999;18(30):4313–25.
Duch A, de Nadal E, Posas F. The p38 and Hog1 SAPKs control cell cycle progression in response to environmental stresses. FEBS Lett. 2012;586(18):2925–31.
Joaquin M, Gubern A, Gonzalez-Nunez D, Josue Ruiz E, Ferreiro I, de Nadal E, et al. The p57 CDKi integrates stress signals into cell-cycle progression to promote cell survival upon stress. EMBO J. 2012;31(13):2952–64.
Yoshizuka N, Chen RM, Xu Z, Liao R, Hong L, Hu WY, et al. A novel function of p38-regulated/activated kinase in endothelial cell migration and tumor angiogenesis. Mol Cell Biol. 2012;32(3):606–18.
Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59(2):152–7.
Tokuda H, Kanno Y, Ishisaki A, Takenaka M, Harada A, Kozawa O. Interleukin (IL)-17 enhances tumor necrosis factor-alpha-stimulated IL-6 synthesis via p38 mitogen-activated protein kinase in osteoblasts. J Cell Biochem. 2004;91(5):1053–61.
Westra J, Limburg PC, de Boer P, van Rijswijk MH. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann Rheum Dis. 2004;63(11):1453–9.
Campbell J, Ciesielski CJ, Hunt AE, Horwood NJ, Beech JT, Hayes LA, et al. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004;173(11):6928–37.
Young P, McDonnell P, Dunnington D, Hand A, Laydon J, Lee J. Pyridinyl imidazoles inhibit IL-1 and TNF production at the protein level. Agents Actions. 1993;39 Spec No:C67–9.
Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol. 1999;162(9):5367–73.
Chae HJ, Chae SW, Chin HY, Bang BG, Cho SB, Han KS, et al. The p38 mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumor necrosis factor in osteoblasts. Bone. 2001;28(1):45–53.
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(2):282–90.
Rossa C, Ehmann K, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells. J Interferon Cytokine Res. 2006;26(10):719–29.
Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145(10):3297–303.
Thouverey C, Caverzasio J. The p38alpha MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell Mol Life Sci. 2012;69(18):3115–25.
Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem. 2000;275(40):31155–61.
McLay LM, Halley F, Souness JE, McKenna J, Benning V, Birrell M, et al. The discovery of RPR 200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg Med Chem. 2001;9(2):537–54.
Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, et al. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther. 2006;317(3):1044–53.
Medicherla S, Ma JY, Mangadu R, Jiang Y, Zhao JJ, Almirez R, et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318(1):132–41.
Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, Yoshikawa H. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthr Rheum. 2003;48(9):2670–81.
Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthr Rheum. 2000;43(1):175–83.
Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthr Rheum. 2006;54(9):2745–56.
Fijen JW, Zijlstra JG, De Boer P, Spanjersberg R, Tervaert JW, Van Der Werf TS, et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin Exp Immunol. 2001;124(1):16–20.
Parasrampuria DA, de Boer P, Desai-Krieger D, Chow AT, Jones CR. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J Clin Pharmacol. 2003;43(4):406–13.
Genovese MC, Cohen SB, Wofsy D, Weinblatt ME, Firestein GS, Brahn E, et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J Rheumatol. 2011;38(5):846–54.
Haddad JJ. VX-745. Vertex pharmaceuticals. Curr Opin Investig Drugs. 2001;2(8):1070–6.
Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthr Rheum. 2009;60(5):1232–41.
Goldstein DM, Kuglstatter A, Lou Y, Soth MJ. Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J Med Chem. 2010;53(6):2345–53.
Schreiber S, Feagan B, D’Haens G, Colombel JF, Geboes K, Yurcov M, et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4(3):325–34.
Hill RJ, Dabbagh K, Phippard D, Li C, Suttmann RT, Welch M, et al. Pamapimod, a novel p38 mitogen-activated protein kinase inhibitor: preclinical analysis of efficacy and selectivity. J Pharmacol Exp Ther. 2008;327(3):610–9.
Cohen SB, Cheng TT, Chindalore V, Damjanov N, Burgos-Vargas R, Delora P, et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthr Rheum. 2009;60(2):335–44.
Lee MR, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem. 2005;12(25):2979–94.
Fabbro D, Ruetz S, Buchdunger E, Cowan-Jacob SW, Fendrich G, Liebetanz J, et al. Protein kinases as targets for anticancer agents: from inhibitors to useful drugs. Pharmacol Ther. 2002;93(2–3):79–98.
Fischer S, Koeberle SC, Laufer SA. p38alpha mitogen-activated protein kinase inhibitors, a patent review (2005–2011). Expert Opin Ther Pat. 2011;21(12):1843–66.
Angell RM, Angell TD, Bamborough P, Bamford MJ, Chung CW, Cockerill SG, et al. Biphenyl amide p38 kinase inhibitors 4: DFG-in and DFG-out binding modes. Bioorg Med Chem Lett. 2008;18(15):4433–7.
Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279(3):1453–61.
Borsch-Haubold AG, Pasquet S, Watson SP. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. J Biol Chem. 1998;273(44):28766–72.
Goldstein DM, Gabriel T. Pathway to the clinic: inhibition of P38 MAP kinase. A review of ten chemotypes selected for development. Curr Top Med Chem. 2005;5(10):1017–29.
Pettus LH, Wurz RP, Xu S, Herberich B, Henkle B, Liu Q, et al. Discovery and evaluation of 7-alkyl-1,5-bis-aryl-pyrazolopyridinones as highly potent, selective, and orally efficacious inhibitors of p38alpha mitogen-activated protein kinase. J Med Chem. 2010;53(7):2973–85.
Nikas SN, Drosos AA. SCIO-469 Scios Inc. Curr Opin Investig Drugs. 2004;5(11):1205–12.
Mavunkel BJ, Chakravarty S, Perumattam JJ, Luedtke GR, Liang X, Lim D, et al. Indole-based heterocyclic inhibitors of p38alpha MAP kinase: designing a conformationally restricted analogue. Bioorg Med Chem Lett. 2003;13(18):3087–90.
Genovese MC. Inhibition of p38: has the fat lady sung? Arthr Rheum. 2009;60(2):317–20.
Schieven GL. The p38alpha kinase plays a central role in inflammation. Curr Top Med Chem. 2009;9(11):1038–48.
Tong SE, Daniels SE, Black P, Chang S, Protter A, Desjardins PJ. Novel p38alpha mitogen-activated protein kinase inhibitor shows analgesic efficacy in acute postsurgical dental pain. J Clin Pharmacol. 2012;52(5):717–28.
Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86(6):1534–44.
Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33.
Weisman MH. Newly diagnosed rheumatoid arthritis. Ann Rheum Dis. 2002;61(4):287–9.
Karcher SC, Laufer SA. Successful structure-based design of recent p38 MAP kinase inhibitors. Curr Top Med Chem. 2009;9(7):655–76.
Vertex moves to re-allocate resources from VX-745 in p38 MAP kinase program to accelerate development of second generation drug candidates VX-702 and VX-850 (media release). 24 Sep 2012 (online). http://www.prnewswire.com/news-releases/vertex-moves-to-re-allocate-resources-from-vx-745-in-p38-map-kinase-program-to-accelerate-development-of-second-generation-drug-candidates-vx-702-and-vx-850-72104487.html. Accessed 11 Jan 2013.
Vertext pharmaceuticals reports third quarter 2001 financial results (media release). 24 Oct 2001 (online). http://investors.vrtx.com/releasedetail.cfm?releaseid=62799. Accessed 11 Jan 2013.
Vertext pharmaceuticals initiates phase II clinical study in rheumatoid arthritis with investigational oral p38 MAP kinase inhibitor VX-702 (media release). 10 June 2005. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=233067. Accessed 11 Jan 2013.
Isoquinoline inhibitors of p38: EPO patent EP1414455 http://patent.ipexl.com/EP/EP1414455.html. Accessed 11 Jan 2013.
Trejo A, Arzeno H, Browner M, Chanda S, Cheng S, Comer DD, et al. Design and synthesis of 4-azaindoles as inhibitors of p38 MAP kinase. J Med Chem. 2003;46(22):4702–13.
Alten RE, Zerbini C, Jeka S, Irazoque F, Khatib F, Emery P, et al. Efficacy and safety of pamapimod in patients with active rheumatoid arthritis receiving stable methotrexate therapy. Ann Rheum Dis. 2010;69(2):364–7.
Aston NM, Bamborough P, Buckton JB, Edwards CD, Holmes DS, Jones KL, et al. p38alpha mitogen-activated protein kinase inhibitors: optimization of a series of biphenylamides to give a molecule suitable for clinical progression. J Med Chem. 2009;52(20):6257–69.
Ostenfeld T, Krishen A, Lai RY, Bullman J, Baines AJ, Green J, Anand P, Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. Eur J Pain. 2012. doi:10.1002/j.1532-2149.2012.00256.x.
GlaxoSmithKline. Result summaries: losmapimod. http://www.gsk-clinicalstudyregister.com/result_comp_list.jsp?phase=All&studyType=All&population=All&marketing=No&compound=losmapimod. Accessed 11 Jan 2013.
Kim C, Cheng CY, Saldanha SA, Taylor SS. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell. 2007;130(6):1032–43.
Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9(9):1019–27.
Cheung PC, Campbell DG, Nebreda AR, Cohen P. Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 2003;22(21):5793–805.
Opar A. Kinase inhibitors attract attention as oral rheumatoid arthritis drugs. Nat Rev Drug Discov. 2010;9(4):257–8.
Koeberle SC, Romir J, Fischer S, Koeberle A, Schattel V, Albrecht W, et al. Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol. 2012;8(2):141–3.
Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69(Suppl. 1):i77–82.
Bonilla-Hernan MG, Miranda-Carus ME, Martin-Mola E. New drugs beyond biologics in rheumatoid arthritis: the kinase inhibitors. Rheumatology (Oxford). 2011;50(9):1542–50.
Schieven GL, Zhang RF, Pitt S, Shen DR, Cao J, Sack J, et al. BMS-582949 is a dual action p38 kinase inhibitor well suited to avoid resistance mechanisms that increase p38 activation in cells. Arthr Rheum. 2010;62(10 Suppl.):S629–30.
Genovese MC, Gao L, Yin J, Smith S, Weinblatt ME, Smolen JS, et al. Proof of concept study for a potent p38 MAPK dual action inhibitor BMS-582949 in subjects with RA receiving concomitant methotrexate. Arthr Rheum. 2010;62(10 Suppl.):S469–70.
Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8(6):480–99.
Guma M, Hammaker D, Topolewski K, Corr M, Boyle DL, Karin M, et al. Antiinflammatory functions of p38 in mouse models of rheumatoid arthritis: advantages of targeting upstream kinases MKK-3 or MKKk-6. Arthr Rheum. 2012;64(9):2887–95.
Hammaker D, Boyle DL, Firestein GS. Synoviocyte innate immune responses: TANK-binding kinase-1 as a potential therapeutic target in rheumatoid arthritis. Rheumatology (Oxford). 2012;51(4):610–8.
Engstrom W, Ward A, Moorwood K. The role of scaffold proteins in JNK signalling. Cell Prolif. 2010;43(1):56–66.
Gallagher TF, Seibel GL, Kassis S, Laydon JT, Blumenthal MJ, Lee JC, et al. Regulation of stress-induced cytokine production by pyridinylimidazoles; inhibition of CSBP kinase. Bioorg Med Chem. 1997;5(1):49–64.
Liverton NJ, Butcher JW, Claiborne CF, Claremon DA, Libby BE, Nguyen KT, et al. Design and synthesis of potent, selective, and orally bioavailable tetrasubstituted imidazole inhibitors of p38 mitogen-activated protein kinase. J Med Chem. 1999;42(12):2180–90.
Koeberle SC, Fischer S, Schollmeyer D, Schattel V, Grutter C, Rauh D, et al. Design, synthesis, and biological evaluation of novel disubstituted dibenzosuberones as highly potent and selective inhibitors of p38 mitogen activated protein kinase. J Med Chem. 2012;55(12):5868–77.
Liu C, Lin J, Wrobleski ST, Lin S, Hynes J, Wu H, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][ 1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38alpha MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53(18):6629–39.
Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24(6):1185–94.
Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–49.
Acknowledgments
Both authors planned, drafted and revised the manuscript in collaboration. They thank the UK Medical Research Council for their support and declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paunovic, V., Harnett, M.M. Mitogen-Activated Protein Kinases as Therapeutic Targets for Rheumatoid Arthritis. Drugs 73, 101–115 (2013). https://doi.org/10.1007/s40265-013-0014-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0014-6