Abstract
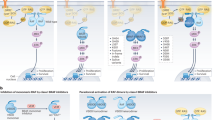
The mitogen activated protein kinase/extracellular signal-related kinase (MAPK/ERK) signaling pathway serves an integral role in growth, proliferation, differentiation, migration, and survival of all mammalian cells. Aberrant signaling of this pathway is often observed in several types of hematologic and solid malignancies. The most frequent insult to this signaling cascade, leading to its constitutive activation, is to the serine/threonine kinase rapidly accelerating fibrosarcoma (RAF). Considering this, the development and approval of various small-molecule inhibitors targeting the MAPK/ERK pathway has become a mainstay of treatment as either mono- or combination therapy in these cancers. Although effective initially, a major clinical barrier with these inhibitors is the relapse of patients due to drug resistance. Knowledge of the mechanisms of resistance to these drugs is still premature, highlighting the need for a more in-depth understanding of how patients become insensitive to these pharmacologic interventions. Herein, we will succinctly summarize the milestones in the approval of select MAPK/ERK pathway inhibitors, their use in patients, and major modes of resistance.

Similar content being viewed by others
References
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signaling pathways in cancer. Oncogene. 2007;26:3279–90.
Robert PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Thargets. 2012;16(1):15–31.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–67.
Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
Akbani R, Cancer Genome Atlas Network et al. Genomic classification of cutaneous melanoma. Cell 2015;161:1681–1696.
Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Clin Med Assoc. 2010;73(3):113–28.
Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015;6(6):660–7.
Yarchoan M, LiVolsi VA, Brose MS. BRAF mutation and thyroid cancer recurrence. Clin Oncol. 2015;33(1):7–8.
Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–15.
Ragad T. Targeting RTK signaling pathways in cancer. Cancers (Basel). 2015;7(3):1758–84.
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19.
Ribas A, Kim KB, Schuchter LM, et al. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAFV600E mutation-positive melanoma. J Clin Oncol. 2011;29(Suppl):8509.
Zhang C, Spevak W, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature. 2015;526:583–6.
Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–15.
Oberholzer PA, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–21.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16.
Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373:1733–47.
Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–21.
Hatzivassilious G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to active the MAPK pathway and enhance growth. Nature. 2010;464:431–5.
Holderfield M, Merritt H, Chan J, et al. RAF inhibitors active the MAPK pathway by relieving inhibitory autophosphorylation. Cell. 2013;23(5):594–602.
Falchoook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumors: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901.
Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013;31(26):3205–11.
Hauschild A, Grob JJ, Demidov LV, et al. Debrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label phase 3 randomized controlled trial. Lancet. 2012;380(9839):358–65.
Delord JP, Robert C, Nyakas M, et al. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Can Res. 2017;23(18):5339–48.
US FDA hematology/oncology (cancer) approvals and safety notifications. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Accessed Sept 2016.
Ali SM, He J, Carson W, et al. Extended antitumor response of a BRAF V600E papillary thyroid carcinoma to vemurafenib. Case Rep Oncol. 2014;7(2):343–8.
Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med. 2007;356:125–34.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28.
Zhang L, Singh RR, Stingo F, et al. BRAAF kinase domain mutation are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am J Hematol. 2014;89(5):499–504.
Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–9.
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14.
Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–9.
Gencler B, Gonul M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569.
Blumenschein GR Jr, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)dagger. Ann Oncol. 2015;26:894–901.
Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3 K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–9.
Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–65.
FDA approves Cotellic as part of combination treatment for advanced melanoma. In: fda.gov. 2015. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471934.htm. Accessed 17 Dec 2017.
Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23.
Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–11.
Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32.
Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–6.
Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47.
Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA. 2017;317:1844–53.
Awada A, Delord JP, Houede N et al. Safety and recommended phase II dose (RP2D) of the selective oral MEK1/2 inhibitor pimasertib (MSC1936369B/AS703026): results of a phase I trial. Eur J Cancer. 2012;48:6185–6186 (abstract 604).
Macarulla T, Cervantes A, Tabernero J, et al. Phase I study of FOLFIRI plus pimasertib as second-line treatment for KRAS-mutated metastatic colorectal cancer. Br J Cancer. 2015;112(12):1874–81.
Lebbe C, Lesimple T, Kruit W, et al. Pimasertib (PIM) versus dacarbazine (DTIC) in patients (pts) with cutaneous NRAS melanoma: a controlled, open-label phase II trial with crossover. Ann Oncol. 2016;27(6):1136.
Binimetinib (MEK 162). In: arraybiopharma.com. 2017. http://www.arraybiopharma.com/product-pipeline/binimetinib/. Accessed 17 Dec 2017.
Lee PA, Wallace E, Marlow A et al. Preclinical development of ARRY-162, a potent and selective MEK 1/2 inhibitor. Cancer Res. 2010;70:2515 (abstract).
Bendell JC, Javle M, Bekaii-Sabb TS, et al. A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer. 2017;116(5):575–83.
Cho M, Gong J, Frankel P, et al. A phase I clinical trial of binimetinib in combination with FOLFOX in patients with advanced metastatic colorectal cancer who failed prior standard therapy. Oncotarget. 2017;8(45):79750–60.
Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–77.
Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109.
Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30.
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition verus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88.
Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76.
Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 1: A phase 3 trial of encorafenib (enco) plus binimetinib (bini) versus vemurafenib (vem) or enco in BRAF-mutant melanoma. Array BioPharma Inc. Society for Melanoma Research 2016 Congress. Nov 2011. Boston, MA.
Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 2: a phase 3 trial of encorafenib plus binimetinib versus encorafenib in BRAF-mutant melanoma. Array BioPharma Inc. ESMO Congress. 2017. Madrid, Spain.
Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Onc. 2017;14:463–82.
Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6.
Ribas A, Hodi FS, Lawrence D, et al. KEYNOTE-022 update: phase 1 study of first of first -line pembrolizumab plus dabrafenib and trametinib for BRAF-mutant advanced melanoma. ESMO Congress. 2017. Abstract 1216O.
Cui G, Liu D, Li W, et al. A meta-analysis of the association between BRAF mutation and nonsmall cell lung cancer. Medicine (Baltimore). 2017;96:e6552.
Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–93.
Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387:1415–26.
FDA grants regular approval to dabrafenib and trametinib combination for metastatic NSCLC with BRAF V600E mutation. In: fda.gov. 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm564331.htm. Accessed 17 Dec 2017.
Cohen R, Cervera P, Svrcek M, et al. BRAF-mutated colorectal cancer: what is the optimal strategy for treatment? Curr Treat Opt Oncol. 2017;18:9.
Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313–20.
Geng F, Wang Z, Yin H, et al. Molecular targeted drugs and treatment of colorectal cancer: recent progress and future perspectives. Cancer Biother Radiopharm. 2017;32:149–60.
Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res. 2012;72:779–89.
Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6:1352–65.
Kopetz S. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). In: ascopubs.org. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.3505. 2017. Accessed 24 Jul 2017.
Manzano JL, Layos L, Buges C, et al. Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med. 2016;4(12):237.
Solit DB, Rosen N. Resistance to BRAF inhibitors in melanomas. N Engl J Med. 2011;362:772–4.
Mao M, Feng T, Mariadason JM, Tsao CC, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can ber overcome with PI3 K inhibition or demethylating agents. Clin Cancer Res. 2012;19(3):657–67.
Prahallad A, Sun C, Huang S. Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–3.
Halaban R. RAC1 and melanoma. Clin Ther. 2015;37(3):682–5.
Rajendran V, Gopalakrishnan C, Purohit R. Impact of point mutation P29S in RAC1 on tumorigenesis. Tumour Biol. 2016;37(11):15293–304.
Zhou Y, Liao Q, Han Y, et al. Rac1 overexpression is correlated with epithelial mesenchymal transition and predicts poor prognosis in non-small lung cancer. J Cancer. 2016;7(14):2100–9.
Liu B, Xiong J, Liu G, et al. High expression of Rac1 is correlated with partial reversed cell polarity and poor prognosis in invasive ductal carcinoma of the breast. Tumor Biol. 2017;39(7):1010428317710908.
Watson IR, Li L, Cabeceiras PK, et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Can Res. 2014. https://doi.org/10.1158/0008-5472.CAN-14-1232-T.
Stahl JM, Cheung M, Sharma A, et al. Loss of PTEN promotes tumor development in malignant melanoma. Can Res. 2003;63(11):2881–90.
Wu H, Giel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–22.
Kennedy SG, Wagner AJ, Conzen SD, et al. The PI3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–13.
Cheney IW, Johnson DE, Vaillancourt MT, et al. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998;58:2331–4.
Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through suppression of BIM expression. Can Res. 2011;71(7):2750–60.
Gogada R, Yadav N, Liu J, et al. BIM, a proapoptotic protein, upregulated via transcription factor E2F1-deendet mechanism, functions as a prosurvival molecule in cancer. JBC. 2012. https://doi.org/10.1074/jbc.M112.386102.
Catalanotti F, Cheng DT, Shoushtari AN, et al. PTEN loss-of-function alterations are associated with intrinsic resistance to BRAF inhibitors in metastatic melanoma. JCO Precis Oncol. 2017. https://doi.org/10.1200/PO.1600054.
Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res. 2013. https://doi.org/10.1158/1078-0432.CCR-13-0259.
Smalley KS, Lioni M, Dalla M, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7(9):2876–83.
Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361(1):98–9.
Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28(10):3534.
Kulkarni A, Al-Hraishawi H, Simhadri S, et al. BRAF fusion as a novel mechanism of acquired resistance to vemurafenib in BRAFv600E mutant melanomas. Biol Hum Tumors. 2017. https://doi.org/10.1158/1078-0432.CCR-16-0758.
Kim HS, Jung M, Kang HN, et al. Oncogenic BRAF fusions in mucosal melanomas active the MAPK pathway and are sensitive to MEK/PI3K inhibition or MEK/CDK4/6 inhibition.
Emery CM, Vijayendran KG, Zipser MC. MEK1 mutations confer resistance to MEK and BRAF inhibition. PNAS. 2009. https://doi.org/10.1073/pnas.0905833106.
Johannessen CM, Boehm JS, Kim SY. COT/MAP3K8 drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–72.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82.
O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30.
Goel HL, Mercurio AM. VEGF targets the tumor cell. Nat Rev Cancer. 2013;13:871–82.
Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to BRAF(V600E) inhibition by RTK or NRAS upregulation. Nature. 2010;468(7326):973–7.
Shi H, Kong X, Ribas A, et al. Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cell to BRAF(V600E) inhibition. Cancer Res. 2011;71:5067–74.
Perna D, Karreth FA, Rust AG. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. PNAS. 2015. https://doi.org/10.1073/pnas.1418163112.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Paraiso KHT, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–60.
Sweetlove M, Wrightson E, Kolekar S, et al. Inhibitors of pan-PI3 K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol. 2015;5:135.
Temraz S, Mukerji D, Shamseddine A. Dual inhibition of MEK and PI3K pathway in KRAS and BRAF mutated colorectal cancers. Int J Mol Sci. 2015;16(9):22976–88.
Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 2017;18:345–60.
Miyata Y, Nakamoto H, Necker L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19(3):347–65.
Pacey S, Gore M, Chao D, et al. A phase II trial of 17-allylamino, 17-demthoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2012;30(1):341–9.
Byrd KM, Subramanian C, Sanchez J, et al. Synthesis and biological evaluation of Novobiocin core analogues as Hsp90 inhibitors. Chem Eur J. 2016;22:6921–31.
White PT, Subramanian C, Zhu Q, et al. Novel Hsp90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery. 2016;159(1):142–51.
Raveendran S, Rao A, and Storkus W. Combination immunotherapy of melanoma by inhibiting HSP90 and targeting its client proteins (TUM7P.934). J Immunol. 2014;192:1 Supplemental 203.16.
Chai RC, Vieusseux JL, Lang BJ, et al. Histone deacteylase activity mediates acquired resistance towards structurally diverse hsp90 inhibitors. Mol Oncol. 2017;11(5):567–83.
Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95.
National Comprehensive Cancer Network. Melanoma NCCN Guidelines with NCCN Evidence Blocks. In: nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/melanoma_blocks.pdf. 2017. Accessed 24 Jul 2017.
National Comprehensive Cancer Network. Thyroid Carcinoma NCCN Guidelines with NCCN Evidence Blocks. In: nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/thyroid_blocks.pdf. 2017. Accessed 24 Jul 2017.
National Comprehensive Cancer Network. Colon Cancer NCCN Guidelines with NCCN Evidence Blocks. In: nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf. 2017. Accessed 24 Jul 2017.
National Comprehensive Cancer Network. Non-Small Cell Lung Cancer NCCN Guidelines with NCCN Evidence Blocks. In: nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf. 2017. Accessed 24 Jul 2017.
National Comprehensive Cancer Network. Hairy Cell Leukemia NCCN Guidelines with NCCN Evidence Blocks. In: nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf. 2017. Accessed 24 Jul 2017.
Lexicomp. Vemurafenib: Drug Information. In: UpToDate.com. https://www.uptodate.com/contents/vemurafenib-drug-information?source=search_result&search=Vemurafenib&selectedTitle=1~42. 2017. Accessed 24 Jul 2017.
Lexicomp. Dabrafenib: Drug Information. In: UpToDate.com. https://www.uptodate.com/contents/dabrafenib-drug-information?source=search_result&search=dabrafenib&selectedTitle=1~33. 2017. Accessed 24 Jul 2017.
Lexicomp. Sorafenib: Drug Information. In: UpToDate.com. https://www.uptodate.com/contents/sorafenib-drug-information?source=search_result&search=sorafenib&selectedTitle=1~107. 2017. Accessed 24 Jul 2017.
Lexicomp. Trametinib: Drug Information. In: UpToDate.com. https://www.uptodate.com/contents/trametinib-drug-information?source=search_result&search=trametinib&selectedTitle=1~28. 2017. Accessed 24 Jul 2017.
Lexicomp. Cobimetinib: Drug Information. In: UpToDate.com. https://www.uptodate.com/contents/cobimetinib-drug-information?source=search_result&search=cobimetinib&selectedTitle=1~18. 2017. Accessed 24 Jul 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors would like to acknowledge the following sources of funding that supported in part the work and effort completed on the manuscript. These include funding from the Department of Surgery at the University of Michigan (MSC and TW) as well as grant funding from the National Institute of Health (R01CA120458 and R01CA216919—MSC) and (T32GM007767—JNS).
Conflict of interest
MSC has received consulting fees from EEPI and Acousys Biomedical Devices LLC in the last year—unrelated to the current manuscript. He is cofounder of NanoPharm LLC, and MedGuider LLC and currently serves as CEO of MedGuider. He has ownership interest in both companies, but no royalties have been generated from either company and neither company is related at all to the topic in the manuscript. The other authors declare no conflict of interest.
Additional information
Jaquelyn N. Sanchez and Ton Wang contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Sanchez, J.N., Wang, T. & Cohen, M.S. BRAF and MEK Inhibitors: Use and Resistance in BRAF-Mutated Cancers. Drugs 78, 549–566 (2018). https://doi.org/10.1007/s40265-018-0884-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0884-8